Paclitaxel promotes differentiation of myeloid-derived suppressor cells into dendritic cells in vitro in a TLR4-independent manner
- PMID: 22283566
- PMCID: PMC3386478
- DOI: 10.3109/1547691X.2011.642418
Paclitaxel promotes differentiation of myeloid-derived suppressor cells into dendritic cells in vitro in a TLR4-independent manner
Abstract
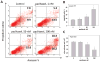
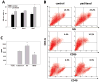
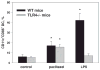
Myeloid cells play a key role in the outcome of anti-tumor immunity and response to anti-cancer therapy, since in the tumor microenvironment they may exert both stimulatory and inhibitory pressures on the proliferative, angiogenic, metastatic, and immunomodulating potential of tumor cells. Therefore, understanding the mechanisms of myeloid regulatory cell differentiation is critical for developing strategies for the therapeutic reversal of myeloid derived suppressor cell (MDSC) accumulation in the tumor-bearing hosts. Here, using an in vitro model system, several potential mechanisms of the direct effect of paclitaxel on MDSC were tested, which might be responsible for the anti-tumor potential of low-dose paclitaxel therapy in mice. It was hypothesized that a decreased level of MDSC in vivo after paclitaxel administration might be due to (i) the blockage of MDSC generation, (ii) an induction of MDSC apoptosis, or (iii) the stimulation of MDSC differentiation. The results revealed that paclitaxel in ultra-low concentrations neither increased MDSC apoptosis nor blocked MDSC generation, but stimulated MDSC differentiation towards dendritic cells. This effect of paclitaxel was TLR4-independent since it was not diminished in cell cultures originated from TLR4-/- mice. These results support a new concept that certain chemotherapeutic agents in ultra-low non-cytotoxic doses may suppress tumor progression by targeting several cell populations in the tumor microenvironment, including MDSC.
Conflict of interest statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Figures

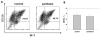
Similar articles
-
Origin and pharmacological modulation of tumor-associated regulatory dendritic cells.Int J Cancer. 2014 Jun 1;134(11):2633-45. doi: 10.1002/ijc.28590. Epub 2014 Jan 20. Int J Cancer. 2014. PMID: 24443321 Free PMC article.
-
Cross-talk between myeloid-derived suppressor cells (MDSC), macrophages, and dendritic cells enhances tumor-induced immune suppression.Semin Cancer Biol. 2012 Aug;22(4):275-81. doi: 10.1016/j.semcancer.2012.01.011. Epub 2012 Feb 1. Semin Cancer Biol. 2012. PMID: 22313874 Free PMC article. Review.
-
Application of paclitaxel in low non-cytotoxic doses supports vaccination with melanoma antigens in normal mice.J Immunotoxicol. 2012 Jul-Sep;9(3):275-81. doi: 10.3109/1547691X.2012.655343. Epub 2012 Mar 27. J Immunotoxicol. 2012. PMID: 22449053
-
Adjuvants and myeloid-derived suppressor cells: enemies or allies in therapeutic cancer vaccination.Hum Vaccin Immunother. 2014;10(11):3251-60. doi: 10.4161/hv.29847. Hum Vaccin Immunother. 2014. PMID: 25483674 Free PMC article. Review.
-
CpG blocks immunosuppression by myeloid-derived suppressor cells in tumor-bearing mice.Clin Cancer Res. 2011 Apr 1;17(7):1765-75. doi: 10.1158/1078-0432.CCR-10-2672. Epub 2011 Jan 13. Clin Cancer Res. 2011. PMID: 21233400
Cited by
-
Myeloid-derived suppressor cells: an emerging target for anticancer immunotherapy.Mol Cancer. 2022 Sep 26;21(1):184. doi: 10.1186/s12943-022-01657-y. Mol Cancer. 2022. PMID: 36163047 Free PMC article. Review.
-
PIKfyve, expressed by CD11c-positive cells, controls tumor immunity.Nat Commun. 2024 Jun 28;15(1):5487. doi: 10.1038/s41467-024-48931-9. Nat Commun. 2024. PMID: 38942798 Free PMC article.
-
Combining chemotherapy with CAR-T cell therapy in treating solid tumors.Front Immunol. 2023 Mar 6;14:1140541. doi: 10.3389/fimmu.2023.1140541. eCollection 2023. Front Immunol. 2023. PMID: 36949946 Free PMC article. Review.
-
Immunological Mechanisms of Low and Ultra-Low Dose Cancer Chemotherapy.Cancer Microenviron. 2015 Aug;8(2):57-64. doi: 10.1007/s12307-013-0141-3. Epub 2013 Nov 29. Cancer Microenviron. 2015. PMID: 24293116 Free PMC article.
-
The Role of Myeloid-Derived Suppressor Cells (MDSC) in Cancer Progression.Vaccines (Basel). 2016 Nov 3;4(4):36. doi: 10.3390/vaccines4040036. Vaccines (Basel). 2016. PMID: 27827871 Free PMC article. Review.
References
-
- Almand B, Clark JI, Nikitina E, van Beynen J, English NR, Knight SC, Carbone DP, Gabrilovich DI. Increased production of immature myeloid cells in cancer patients: A mechanism of immunosuppression in cancer. J Immunol. 2001;166:678–689. - PubMed
-
- Apetoh L, Vegran F, Ladoire S, Ghiringhelli F. Restoration of anti-tumor immunity through selective inhibition of myeloid derived suppressor cells by anti-cancer therapies. Curr Mol Med. 2011;11:365–372. - PubMed
-
- Balkir L, Tourkova IL, Makarenkova VP, Shurin GV, Robbins PD, Yin XM, Chatta G, Shurin MR. Comparative analysis of dendritic cells transduced with different anti-apoptotic molecules: Sensitivity to tumor-induced apoptosis. J Gene Med. 2004;6:537–544. - PubMed
-
- Byrd-Leifer CA, Block EF, Takeda K, Akira S, Ding A. The role of MyD88 and TLR4 in the LPS-mimetic activity of Taxol. Eur J Immunol. 2001;31:2448–2457. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
