The loss of renal dendritic cells and activation of host adaptive immunity are long-term effects of ischemia/reperfusion injury following syngeneic kidney transplantation
- PMID: 22278023
- PMCID: PMC3340432
- DOI: 10.1038/ki.2011.458
The loss of renal dendritic cells and activation of host adaptive immunity are long-term effects of ischemia/reperfusion injury following syngeneic kidney transplantation
Abstract
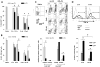
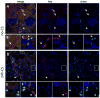
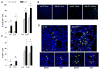
Ischemia/reperfusion injury associated with kidney transplantation induces profound acute injury, influences early graft function, and affects long-term graft outcomes. To determine whether renal dendritic cells play any role during initial innate ischemia/reperfusion injury and the subsequent development of adaptive immune responses, we studied the behavior and function of renal graft and host infiltrating dendritic cells during early and late phases of renal ischemia/reperfusion injury. Wild type to green fluorescent protein (GFP) transgenic rat kidney transplantation was performed with and without 24-h cold storage. Ischemia/reperfusion injury in cold-stored grafts resulted in histopathological changes of interstitial fibrosis and tubular atrophy by 10 weeks, accompanied by upregulation of mRNAs of mediators of interstitial fibrosis and inflammation. In normal rat kidneys, we identified two populations of renal dendritic cells, predominant CD103(-)CD11b/c(+) and minor CD103(+)CD11b/c(+) cells. After transplantation without cold storage, grafts maintained CD103(-) but not CD103(+) GFP-negative renal dendritic cells for 10 weeks. In contrast, both cell subsets disappeared from cold-stored grafts, which associated with a significant GFP-expressing host CD11b/c(+) cell infiltration that included CD103(+) dendritic cells with a TNF-α-producing phenotype. These changes in graft/host dendritic cell populations were associated with progressive infiltration of host CD4(+) T cells with effector/effector-memory phenotypes and IFN-γ secretion. Thus, renal graft ischemia/reperfusion injury caused graft dendritic cell loss and was associated with progressive host dendritic cell and T-cell recruitment. Renal-resident dendritic cells might function as a protective regulatory network.
Figures





Similar articles
-
Transplantation-Induced Ischemia-Reperfusion Injury Modulates Antigen Presentation by Donor Renal CD11c+F4/80+ Macrophages through IL-1R8 Regulation.J Am Soc Nephrol. 2020 Mar;31(3):517-531. doi: 10.1681/ASN.2019080778. Epub 2020 Jan 27. J Am Soc Nephrol. 2020. PMID: 31988271 Free PMC article.
-
CD11c(+) CD103(+) cells of Mycobacterium tuberculosis-infected C57BL/6 but not of BALB/c mice induce a high frequency of interferon-γ- or interleukin-17-producing CD4(+) cells.Immunology. 2015 Apr;144(4):574-86. doi: 10.1111/imm.12411. Immunology. 2015. PMID: 25322675 Free PMC article.
-
NKT cell activation mediates neutrophil IFN-gamma production and renal ischemia-reperfusion injury.J Immunol. 2007 May 1;178(9):5899-911. doi: 10.4049/jimmunol.178.9.5899. J Immunol. 2007. PMID: 17442974
-
Role of Toll-like receptor-4 in renal graft ischemia-reperfusion injury.Am J Physiol Renal Physiol. 2014 Apr 15;306(8):F801-11. doi: 10.1152/ajprenal.00469.2013. Epub 2014 Feb 12. Am J Physiol Renal Physiol. 2014. PMID: 24523386 Free PMC article. Review.
-
The effect of ischemia/reperfusion on the kidney graft.Curr Opin Organ Transplant. 2014 Aug;19(4):395-400. doi: 10.1097/MOT.0000000000000090. Curr Opin Organ Transplant. 2014. PMID: 24905021 Review.
Cited by
-
Sterile Pancreas Inflammation during Preservation and after Transplantation.Int J Mol Sci. 2023 Feb 27;24(5):4636. doi: 10.3390/ijms24054636. Int J Mol Sci. 2023. PMID: 36902067 Free PMC article. Review.
-
Murine cytomegalovirus dissemination but not reactivation in donor-positive/recipient-negative allogeneic kidney transplantation can be effectively prevented by transplant immune tolerance.Kidney Int. 2020 Jul;98(1):147-158. doi: 10.1016/j.kint.2020.01.034. Epub 2020 Feb 21. Kidney Int. 2020. PMID: 32471635 Free PMC article.
-
Dendritic cells and macrophages in the kidney: a spectrum of good and evil.Nat Rev Nephrol. 2014 Nov;10(11):625-43. doi: 10.1038/nrneph.2014.170. Epub 2014 Sep 30. Nat Rev Nephrol. 2014. PMID: 25266210 Free PMC article. Review.
-
The role of dendritic cells regulated by HMGB1/TLR4 signalling pathway in myocardial ischaemia reperfusion injury.J Cell Mol Med. 2019 Apr;23(4):2849-2862. doi: 10.1111/jcmm.14192. Epub 2019 Feb 19. J Cell Mol Med. 2019. PMID: 30784177 Free PMC article.
-
Ischemia/reperfusion Injury and its Consequences on Immunity and Inflammation.Curr Transplant Rep. 2014 Sep 1;1(3):147-154. doi: 10.1007/s40472-014-0017-6. Curr Transplant Rep. 2014. PMID: 25419507 Free PMC article.
References
-
- Meier-Kriesche HU, Schold JD, Srinivas TR, Kaplan B. Lack of improvement in renal allograft survival despite a marked decrease in acute rejection rates over the most recent era. Am J Transplant. 2004 Mar;4(3):378–83. - PubMed
-
- Kasiske BL, Gaston RS, Gourishankar S, Halloran PF, Matas AJ, Jeffery J, et al. Long-term deterioration of kidney allograft function. Am J Transplant. 2005 Jun;5(6):1405–14. - PubMed
-
- Joosten SA, Sijpkens YW, van Kooten C, Paul LC. Chronic renal allograft rejection: pathophysiologic considerations. Kidney Int. 2005 Jul;68(1):1–13. - PubMed
-
- Paul LC. Chronic renal transplant loss. Kidney Int. 1995 Jun;47(6):1491–9. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

