Cytosolic Bax: does it require binding proteins to keep its pro-apoptotic activity in check?
- PMID: 22277657
- PMCID: PMC3308772
- DOI: 10.1074/jbc.M111.248906
Cytosolic Bax: does it require binding proteins to keep its pro-apoptotic activity in check?
Abstract
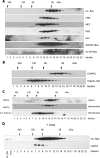
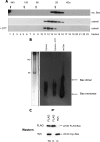
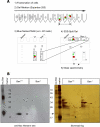
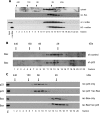
Bax is kept inactive in the cytosol by refolding its C-terminal transmembrane domain into the hydrophobic binding pocket. Although energetic calculations predicted this conformation to be stable, numerous Bax binding proteins were reported and suggested to further stabilize inactive Bax. Unfortunately, most of them have not been validated in a physiological context on the endogenous level. Here we use gel filtration analysis of the cytosol of primary and established cells to show that endogenous, inactive Bax runs 20-30 kDa higher than recombinant Bax, suggesting Bax dimerization or the binding of a small protein. Dimerization was excluded by a lack of interaction of differentially tagged Bax proteins and by comparing the sizes of dimerized recombinant Bax with cytosolic Bax on blue native gels. Surprisingly, when analyzing cytosolic Bax complexes by high sensitivity mass spectrometry after anti-Bax immunoprecipitation or consecutive purification by gel filtration and blue native gel electrophoresis, we detected only one protein, called p23 hsp90 co-chaperone, which consistently and specifically co-purified with Bax. However, this protein could not be validated as a crucial inhibitory Bax binding partner as its over- or underexpression did not show any apoptosis defects. By contrast, cytosolic Bax exhibits a slight molecular mass shift on SDS-PAGE as compared with recombinant Bax, which suggests a posttranslational modification and/or a structural difference between the two proteins. We propose that in most healthy cells, cytosolic endogenous Bax is a monomeric protein that does not necessarily need a binding partner to keep its pro-apoptotic activity in check.
Figures



Similar articles
-
An Autoinhibited Dimeric Form of BAX Regulates the BAX Activation Pathway.Mol Cell. 2016 Aug 4;63(3):485-97. doi: 10.1016/j.molcel.2016.06.010. Epub 2016 Jul 14. Mol Cell. 2016. PMID: 27425408 Free PMC article.
-
Conformational rearrangements in the pro-apoptotic protein, Bax, as it inserts into mitochondria: a cellular death switch.J Biol Chem. 2014 Nov 21;289(47):32871-82. doi: 10.1074/jbc.M114.593897. Epub 2014 Oct 14. J Biol Chem. 2014. PMID: 25315775 Free PMC article.
-
The C-terminus of interferon gamma receptor beta chain (IFNgammaR2) has antiapoptotic activity as a Bax inhibitor.Cancer Biol Ther. 2009 Sep;8(18):1771-86. doi: 10.4161/cbt.8.18.9323. Epub 2009 Sep 20. Cancer Biol Ther. 2009. PMID: 19657228 Free PMC article.
-
Structural biology of the Bcl-2 family of proteins.Biochim Biophys Acta. 2004 Mar 1;1644(2-3):83-94. doi: 10.1016/j.bbamcr.2003.08.012. Biochim Biophys Acta. 2004. PMID: 14996493 Review.
-
Multistep and multitask Bax activation.Mitochondrion. 2010 Nov;10(6):604-13. doi: 10.1016/j.mito.2010.08.003. Epub 2010 Aug 10. Mitochondrion. 2010. PMID: 20709625 Review.
Cited by
-
Bax targets mitochondria by distinct mechanisms before or during apoptotic cell death: a requirement for VDAC2 or Bak for efficient Bax apoptotic function.Cell Death Differ. 2014 Dec;21(12):1925-35. doi: 10.1038/cdd.2014.119. Epub 2014 Aug 22. Cell Death Differ. 2014. PMID: 25146925 Free PMC article.
-
Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) protein-induced lysosomal translocation of proapoptotic effectors is mediated by phosphofurin acidic cluster sorting protein-2 (PACS-2).J Biol Chem. 2012 Jul 13;287(29):24427-37. doi: 10.1074/jbc.M112.342238. Epub 2012 May 29. J Biol Chem. 2012. PMID: 22645134 Free PMC article.
-
The porin VDAC2 is the mitochondrial platform for Bax retrotranslocation.Sci Rep. 2016 Sep 13;6:32994. doi: 10.1038/srep32994. Sci Rep. 2016. PMID: 27620692 Free PMC article.
-
Apoptosis induced by the fungal pathogen gliotoxin requires a triple phosphorylation of Bim by JNK.Cell Death Differ. 2013 Oct;20(10):1317-29. doi: 10.1038/cdd.2013.78. Epub 2013 Jul 5. Cell Death Differ. 2013. PMID: 23832115 Free PMC article.
-
EBV BCL-2 homologue BHRF1 drives chemoresistance and lymphomagenesis by inhibiting multiple cellular pro-apoptotic proteins.Cell Death Differ. 2020 May;27(5):1554-1568. doi: 10.1038/s41418-019-0435-1. Epub 2019 Oct 23. Cell Death Differ. 2020. PMID: 31645677 Free PMC article.
References
-
- Youle R. J., Strasser A. (2008) The BCL-2 protein family. Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 9, 47–59 - PubMed
-
- Cheng E. H., Sheiko T. V., Fisher J. K., Craigen W. J., Korsmeyer S. J. (2003) VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science 301, 513–517 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

