Metastasis is an early event in mouse mammary carcinomas and is associated with cells bearing stem cell markers
- PMID: 22277639
- PMCID: PMC3496135
- DOI: 10.1186/bcr3102
Metastasis is an early event in mouse mammary carcinomas and is associated with cells bearing stem cell markers
Abstract
Introduction: It is still uncertain whether metastasis is predominantly an early or late event in tumor progression. The detection of early metastases and cells responsible for the dissemination may therefore have significant clinical implications.
Methods: Lung dissemination and/or metastasis were investigated in mice carrying the polyomavirus middle-T oncogene (PyMT) during different stages of mammary tumorigenesis using the colony forming assay. Immunocytochemical or immunohistochemical staining was used to identify subpopulations of cells responsible for lung dissemination and metastasis. Histological examination was used to show primary and metastatic tumors. The tumor-initiating and metastatic capacity of cells expressing stem cell markers was assessed in syngeneic wild-type (WT) mice whose mammary fat pads were injected with these cells.
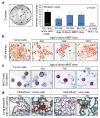
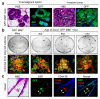
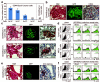
Results: Metastatic mammary epithelial cells were detected in the lungs of mice carrying the PyMT oncogene (MMT mice). These cells were observed early in breast tumorigenesis when the mammary tree appeared by histological inspection to be normal (or at a premalignant stage), suggesting the possession of disseminating and metastatic capacity even before full malignant transformation. Some of the disseminated cells and lung metastases displayed surface stem cell markers. These findings suggest that stem cells from apparently precancerous primary lesions could be a source of metastasis. Indeed, injection of lung tissue cells from MMT mice into syngeneic WT mice resulted in the formation of mammary tumors. These tumors resembled their parent mammary tumors in the MMT donors as well as grafted tumors derived from mammary tumor cells. Furthermore, when we injected lung tissue cells from GFP MMT mice into the fat pads of recipient WT mice, disseminated or metastatic GFP-expressing cells were detected in the lungs, lymph nodes and blood of the recipient WT mice. We finally identified a subpopulation of mammary epithelial/tumor cells expressing CD44 and Sca1 that was largely responsible for dissemination and metastasis in MMT mice.
Conclusions: The tumorigenic and metastatic potential of a subpopulation of mammary epithelial/tumor cells in MMT mice is endowed relatively early in mammary neoplasms and suggests a potential role for cancer stem cell sub-populations in metastasis.
Figures



Similar articles
-
Transformation of enriched mammary cell populations with polyomavirus middle T antigen influences tumor subtype and metastatic potential.Breast Cancer Res. 2015 Oct 1;17(1):132. doi: 10.1186/s13058-015-0641-9. Breast Cancer Res. 2015. PMID: 26429062 Free PMC article.
-
Hypoxia-inducible factor 1α promotes primary tumor growth and tumor-initiating cell activity in breast cancer.Breast Cancer Res. 2012 Jan 7;14(1):R6. doi: 10.1186/bcr3087. Breast Cancer Res. 2012. PMID: 22225988 Free PMC article.
-
Biomarkers of residual disease, disseminated tumor cells, and metastases in the MMTV-PyMT breast cancer model.PLoS One. 2013;8(3):e58183. doi: 10.1371/journal.pone.0058183. Epub 2013 Mar 8. PLoS One. 2013. PMID: 23520493 Free PMC article.
-
The C3(1)/SV40 T-antigen transgenic mouse model of mammary cancer: ductal epithelial cell targeting with multistage progression to carcinoma.Oncogene. 2000 Feb 21;19(8):1020-7. doi: 10.1038/sj.onc.1203280. Oncogene. 2000. PMID: 10713685 Review.
-
Wnt signaling, stem cells, and the cellular origin of breast cancer.Stem Cell Rev. 2007 Jun;3(2):157-68. doi: 10.1007/s12015-007-0025-3. Stem Cell Rev. 2007. PMID: 17873348 Review.
Cited by
-
Heat Shock Proteins Are Essential Components in Transformation and Tumor Progression: Cancer Cell Intrinsic Pathways and Beyond.Int J Mol Sci. 2019 Sep 11;20(18):4507. doi: 10.3390/ijms20184507. Int J Mol Sci. 2019. PMID: 31514477 Free PMC article. Review.
-
Interplay of Stem Cell Characteristics, EMT, and Microtentacles in Circulating Breast Tumor Cells.Cancers (Basel). 2013 Nov 14;5(4):1545-65. doi: 10.3390/cancers5041545. Cancers (Basel). 2013. PMID: 24240660 Free PMC article.
-
Genotoxic stress induces Sca-1-expressing metastatic mammary cancer cells.Mol Oncol. 2018 Aug;12(8):1249-1263. doi: 10.1002/1878-0261.12321. Epub 2018 Jun 13. Mol Oncol. 2018. PMID: 29738110 Free PMC article.
-
S100A10 Has a Critical Regulatory Function in Mammary Tumor Growth and Metastasis: Insights Using MMTV-PyMT Oncomice and Clinical Patient Sample Analysis.Cancers (Basel). 2020 Dec 7;12(12):3673. doi: 10.3390/cancers12123673. Cancers (Basel). 2020. PMID: 33297495 Free PMC article.
-
The Establishment of a Lung Colonization Assay for Circulating Tumor Cell Visualization in Lung Tissues.J Vis Exp. 2018 Jun 16;(136):56761. doi: 10.3791/56761. J Vis Exp. 2018. PMID: 29985344 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

