The influence of single bursts versus single spikes at excitatory dendrodendritic synapses
- PMID: 22277089
- PMCID: PMC4472665
- DOI: 10.1111/j.1460-9568.2011.07978.x
The influence of single bursts versus single spikes at excitatory dendrodendritic synapses
Abstract
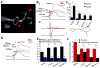
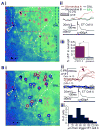
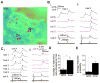
The synchronization of neuronal activity is thought to enhance information processing. There is much evidence supporting rhythmically bursting external tufted cells (ETCs) of the rodent olfactory bulb glomeruli coordinating the activation of glomerular interneurons and mitral cells via dendrodendritic excitation. However, as bursting has variable significance at axodendritic cortical synapses, it is not clear if ETC bursting imparts a specific functional advantage over the preliminary spike in dendrodendritic synaptic networks. To answer this question, we investigated the influence of single ETC bursts and spikes with the in vitro rat olfactory bulb preparation at different levels of processing, via calcium imaging of presynaptic ETC dendrites, dual electrical recording of ETC -interneuron synaptic pairs, and multicellular calcium imaging of ETC-induced population activity. Our findings supported single ETC bursts, versus single spikes, driving robust presynaptic calcium signaling, which in turn was associated with profound extension of the initial monosynaptic spike-driven dendrodendritic excitatory postsynaptic potential. This extension could be driven by either the spike-dependent or spike-independent components of the burst. At the population level, burst-induced excitation was more widespread and reliable compared with single spikes. This further supports the ETC network, in part due to a functional advantage of bursting at excitatory dendrodendritic synapses, coordinating synchronous activity at behaviorally relevant frequencies related to odor processing in vivo.
© 2012 The Authors. European Journal of Neuroscience © 2012 Federation of European Neuroscience Societies and Blackwell Publishing Ltd.
Figures
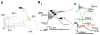


Similar articles
-
Direct Recording of Dendrodendritic Excitation in the Olfactory Bulb: Divergent Properties of Local and External Glutamatergic Inputs Govern Synaptic Integration in Granule Cells.J Neurosci. 2017 Dec 6;37(49):11774-11788. doi: 10.1523/JNEUROSCI.2033-17.2017. Epub 2017 Oct 24. J Neurosci. 2017. PMID: 29066560 Free PMC article.
-
Functional Specialization of Interneuron Dendrites: Identification of Action Potential Initiation Zone in Axonless Olfactory Bulb Granule Cells.J Neurosci. 2019 Dec 4;39(49):9674-9688. doi: 10.1523/JNEUROSCI.1763-19.2019. Epub 2019 Oct 29. J Neurosci. 2019. PMID: 31662426 Free PMC article.
-
Calcium permeable AMPA receptors and autoreceptors in external tufted cells of rat olfactory bulb.Neuroscience. 2007 Feb 9;144(3):1094-108. doi: 10.1016/j.neuroscience.2006.10.041. Epub 2006 Dec 6. Neuroscience. 2007. PMID: 17156930 Free PMC article.
-
From single cells and single columns to cortical networks: dendritic excitability, coincidence detection and synaptic transmission in brain slices and brains.Exp Physiol. 2017 May 1;102(5):489-521. doi: 10.1113/EP085776. Epub 2017 Apr 21. Exp Physiol. 2017. PMID: 28139019 Free PMC article. Review.
-
Dendritic processing within olfactory bulb circuits.Trends Neurosci. 2003 Sep;26(9):501-6. doi: 10.1016/S0166-2236(03)00228-5. Trends Neurosci. 2003. PMID: 12948662 Review.
Cited by
-
Serotonin increases synaptic activity in olfactory bulb glomeruli.J Neurophysiol. 2016 Mar;115(3):1208-19. doi: 10.1152/jn.00847.2015. Epub 2015 Dec 9. J Neurophysiol. 2016. PMID: 26655822 Free PMC article.
-
Modeling Patient-Derived Glioblastoma with Cerebral Organoids.Cell Rep. 2019 Mar 19;26(12):3203-3211.e5. doi: 10.1016/j.celrep.2019.02.063. Cell Rep. 2019. PMID: 30893594 Free PMC article.
-
Quantitative Association of Anatomical and Functional Classes of Olfactory Bulb Neurons.J Neurosci. 2018 Aug 15;38(33):7204-7220. doi: 10.1523/JNEUROSCI.0303-18.2018. Epub 2018 Jul 5. J Neurosci. 2018. PMID: 29976625 Free PMC article.
References
-
- Abbott LF, Regehr WG. Synaptic computation. Nature. 2004;431:796–803. - PubMed
-
- Aroniadou-Anderjaska V, Zhou FM, Priest CA, Ennis M, Shipley MT. Tonic and synaptically evoked presynaptic inhibition of sensory input to the rat olfactory bulb via GABA(B) heteroreceptors. J Neurophysiol. 2000;84:1194–1203. - PubMed
-
- Cadetti L, Belluzzi O. Hyperpolarisation-activated current in glomerular cells of the rat olfactory bulb. Neuroreport. 2001;12:3117–3120. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

