Protein kinase D1 maintains the epithelial phenotype by inducing a DNA-bound, inactive SNAI1 transcriptional repressor complex
- PMID: 22276203
- PMCID: PMC3262827
- DOI: 10.1371/journal.pone.0030459
Protein kinase D1 maintains the epithelial phenotype by inducing a DNA-bound, inactive SNAI1 transcriptional repressor complex
Abstract
Background: Protein kinase D1 is downregulated in its expression in invasive ductal carcinoma of the breast and in invasive breast cancer cells, but its functions in normal breast epithelial cells is largely unknown. The epithelial phenotype is maintained by cell-cell junctions formed by E-cadherin. In cancer cells loss of E-cadherin expression contributes to an invasive phenotype. This can be mediated by SNAI1, a transcriptional repressor for E-cadherin that contributes to epithelial-to-mesenchymal transition (EMT).
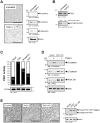
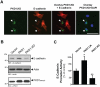
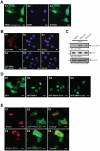
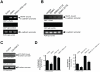
Methodology/principal findings: Here we show that PKD1 in normal murine mammary gland (NMuMG) epithelial cells is constitutively-active in its basal state and prevents a transition to a mesenchymal phenotype. Investigation of the involved mechanism suggested that PKD1 regulates the expression of E-cadherin at the promoter level through direct phosphorylation of the transcriptional repressor SNAI1. PKD1-mediated phosphorylation of SNAI1 occurs in the nucleus and generates a nuclear, inactive DNA/SNAI1 complex that shows decreased interaction with its co-repressor Ajuba. Analysis of human tissue samples with a newly-generated phosphospecific antibody for PKD1-phosphorylated SNAI1 showed that regulation of SNAI1 through PKD1 occurs in vivo in normal breast ductal tissue and is decreased or lost in invasive ductal carcinoma.
Conclusions/significance: Our data describe a mechanism of how PKD1 maintains the breast epithelial phenotype. Moreover, they suggest, that the analysis of breast tissue for PKD-mediated phosphorylation of SNAI1 using our novel phosphoS11-SNAI1-specific antibody may allow predicting the invasive potential of breast cancer cells.
Conflict of interest statement
Figures


Similar articles
-
Protein kinase D1 suppresses epithelial-to-mesenchymal transition through phosphorylation of snail.Cancer Res. 2010 Oct 15;70(20):7810-9. doi: 10.1158/0008-5472.CAN-09-4481. Epub 2010 Oct 12. Cancer Res. 2010. PMID: 20940406
-
SNAI1 is critical for the aggressiveness of prostate cancer cells with low E-cadherin.Mol Cancer. 2014 Feb 24;13:37. doi: 10.1186/1476-4598-13-37. Mol Cancer. 2014. PMID: 24565133 Free PMC article.
-
Requirement of the histone demethylase LSD1 in Snai1-mediated transcriptional repression during epithelial-mesenchymal transition.Oncogene. 2010 Sep 2;29(35):4896-904. doi: 10.1038/onc.2010.234. Epub 2010 Jun 21. Oncogene. 2010. PMID: 20562920 Free PMC article.
-
FoxM1 overexpression promotes epithelial-mesenchymal transition and metastasis of hepatocellular carcinoma.World J Gastroenterol. 2015 Jan 7;21(1):196-213. doi: 10.3748/wjg.v21.i1.196. World J Gastroenterol. 2015. PMID: 25574092 Free PMC article.
-
Role of glycogen synthase kinase-3 in cell fate and epithelial-mesenchymal transitions.Cells Tissues Organs. 2007;185(1-3):73-84. doi: 10.1159/000101306. Cells Tissues Organs. 2007. PMID: 17587811 Review.
Cited by
-
Protein kinase D1 attenuates tumorigenesis in colon cancer by modulating β-catenin/T cell factor activity.Oncotarget. 2014 Aug 30;5(16):6867-84. doi: 10.18632/oncotarget.2277. Oncotarget. 2014. PMID: 25149539 Free PMC article.
-
Association between Single Nucleotide Polymorphisms of PRKD1 and KCNQ3 Gene and Milk Quality Traits in Gannan Yak (Bos grunniens).Foods. 2024 Mar 2;13(5):781. doi: 10.3390/foods13050781. Foods. 2024. PMID: 38472894 Free PMC article.
-
Snai2 and Snai3 transcriptionally regulate cellular fitness and functionality of T cell lineages through distinct gene programs.Immunobiology. 2016 May;221(5):618-33. doi: 10.1016/j.imbio.2016.01.007. Epub 2016 Jan 22. Immunobiology. 2016. PMID: 26831822 Free PMC article.
-
The Post-Translational Regulation of Epithelial-Mesenchymal Transition-Inducing Transcription Factors in Cancer Metastasis.Int J Mol Sci. 2021 Mar 30;22(7):3591. doi: 10.3390/ijms22073591. Int J Mol Sci. 2021. PMID: 33808323 Free PMC article. Review.
-
Regulation of the protein stability of EMT transcription factors.Cell Adh Migr. 2014;8(4):418-28. doi: 10.4161/19336918.2014.969998. Cell Adh Migr. 2014. PMID: 25482633 Free PMC article. Review.
References
-
- Peinado H, Olmeda D, Cano A. Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer. 2007;7:415–428. - PubMed
-
- Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, et al. Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res. 2008;68:3645–3654. - PubMed
-
- Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–558. - PubMed
-
- Barrallo-Gimeno A, Nieto MA. The Snail genes as inducers of cell movement and survival: implications in development and cancer. Development. 2005;132:3151–3161. - PubMed
-
- Batlle E, Sancho E, Franci C, Dominguez D, Monfar M, et al. The transcription factor snail is a repressor of E-cadherin gene expression in epithelial tumour cells. Nat Cell Biol. 2000;2:84–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

