Swelling-activated anion channels are essential for volume regulation of mouse thymocytes
- PMID: 22272123
- PMCID: PMC3257120
- DOI: 10.3390/ijms12129125
Swelling-activated anion channels are essential for volume regulation of mouse thymocytes
Abstract
Channel-mediated trans-membrane chloride movement is a key process in the active cell volume regulation under osmotic stress in most cells. However, thymocytes were hypothesized to regulate their volume by activating a coupled K-Cl cotransport mechanism. Under the patch-clamp, we found that osmotic swelling activates two types of macroscopic anion conductance with different voltage-dependence and pharmacology. At the single-channel level, we identified two types of events: one corresponded to the maxi-anion channel, and the other one had characteristics of the volume-sensitive outwardly rectifying (VSOR) chloride channel of intermediate conductance. A VSOR inhibitor, phloretin, significantly suppressed both macroscopic VSOR-type conductance and single-channel activity of intermediate amplitude. The maxi-anion channel activity was largely suppressed by Gd(3+) ions but not by phloretin. Surprisingly, [(dihydroindenyl)oxy] alkanoic acid (DIOA), a known antagonist of K-Cl cotransporter, was found to significantly suppress the activity of the VSOR-type single-channel events with no effect on the maxi-anion channels at 10 μM. The regulatory volume decrease (RVD) phase of cellular response to hypotonicity was mildly suppressed by Gd(3+) ions and was completely abolished by phloretin suggesting a major impact of the VSOR chloride channel and modulatory role of the maxi-anion channel. The inhibitory effect of DIOA was also strong, and, most likely, it occurred via blocking the VSOR Cl(-) channels.
Keywords: DIOA; anion channels; phloretin; thymocytes; volume regulation.
Figures

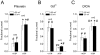

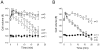
Similar articles
-
Molecular Identities and ATP Release Activities of Two Types of Volume-Regulatory Anion Channels, VSOR and Maxi-Cl.Curr Top Membr. 2018;81:125-176. doi: 10.1016/bs.ctm.2018.07.004. Epub 2018 Aug 17. Curr Top Membr. 2018. PMID: 30243431 Review.
-
Roles of volume-regulatory anion channels, VSOR and Maxi-Cl, in apoptosis, cisplatin resistance, necrosis, ischemic cell death, stroke and myocardial infarction.Curr Top Membr. 2019;83:205-283. doi: 10.1016/bs.ctm.2019.03.001. Epub 2019 Apr 19. Curr Top Membr. 2019. PMID: 31196606 Review.
-
Volume-sensitive anion channels mediate osmosensitive glutathione release from rat thymocytes.PLoS One. 2013;8(1):e55646. doi: 10.1371/journal.pone.0055646. Epub 2013 Jan 30. PLoS One. 2013. PMID: 23383255 Free PMC article.
-
Distinct pharmacological and molecular properties of the acid-sensitive outwardly rectifying (ASOR) anion channel from those of the volume-sensitive outwardly rectifying (VSOR) anion channel.Pflugers Arch. 2016 May;468(5):795-803. doi: 10.1007/s00424-015-1786-1. Epub 2016 Jan 8. Pflugers Arch. 2016. PMID: 26743872
-
Roles of two types of anion channels in glutamate release from mouse astrocytes under ischemic or osmotic stress.Glia. 2006 Oct;54(5):343-57. doi: 10.1002/glia.20400. Glia. 2006. PMID: 16883573
Cited by
-
Chloride in Heart Failure Syndrome: Its Pathophysiologic Role and Therapeutic Implication.Cardiol Ther. 2021 Dec;10(2):407-428. doi: 10.1007/s40119-021-00238-2. Epub 2021 Aug 14. Cardiol Ther. 2021. PMID: 34398440 Free PMC article. Review.
-
The properties, functions, and pathophysiology of maxi-anion channels.Pflugers Arch. 2016 Mar;468(3):405-20. doi: 10.1007/s00424-015-1774-5. Epub 2016 Jan 6. Pflugers Arch. 2016. PMID: 26733413 Review.
-
cAMP-stimulated Cl- secretion is increased by glucocorticoids and inhibited by bumetanide in semicircular canal duct epithelium.BMC Physiol. 2013 Mar 27;13:6. doi: 10.1186/1472-6793-13-6. BMC Physiol. 2013. PMID: 23537040 Free PMC article.
-
Effect of Glycyrrhetic Acid Derivatives on Regulation of Thymocyte Volume.Bull Exp Biol Med. 2023 May;175(1):27-31. doi: 10.1007/s10517-023-05804-3. Epub 2023 Jun 20. Bull Exp Biol Med. 2023. PMID: 37338755
-
Epithelia of the ovine and bovine forestomach express basolateral maxi-anion channels permeable to the anions of short-chain fatty acids.Pflugers Arch. 2014 Sep;466(9):1689-712. doi: 10.1007/s00424-013-1386-x. Epub 2013 Nov 17. Pflugers Arch. 2014. PMID: 24240698
References
-
- Hoffmann E.K., Lambert I.H., Pedersen S.F. Physiology of cell volume regulation in vertebrates. Physiol. Rev. 2009;89:193–277. - PubMed
-
- Okada Y. Ion channels and transporters involved in cell volume regulation and sensor mechanisms. Cell Biochem. Biophys. 2004;41:233–258. - PubMed
-
- Wehner F., Olsen H., Tinel H., Kinne-Saffran E., Kinne R.K. Cell volume regulation: Osmolytes, osmolyte transport, and signal transduction. Rev. Physiol. Biochem. Pharmacol. 2003;148:1–80. - PubMed
-
- Okada Y., Maeno E. Apoptosis, cell volume regulation and volume-regulatory chloride channels. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2001;130:377–383. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

