ETS-1 protein regulates vascular endothelial growth factor-induced matrix metalloproteinase-9 and matrix metalloproteinase-13 expression in human ovarian carcinoma cell line SKOV-3
- PMID: 22270366
- PMCID: PMC3340257
- DOI: 10.1074/jbc.M111.284034
ETS-1 protein regulates vascular endothelial growth factor-induced matrix metalloproteinase-9 and matrix metalloproteinase-13 expression in human ovarian carcinoma cell line SKOV-3
Retraction in
-
Withdrawal: ETS-1 protein regulates vascular endothelial growth factor-induced matrix metalloproteinase-9 and matrix metalloproteinase-13 expression in human ovarian carcinoma cell line SKOV-3.J Biol Chem. 2020 Dec 18;295(51):17887. doi: 10.1074/jbc.W120.016716. J Biol Chem. 2020. PMID: 33454022 Free PMC article. No abstract available.
Abstract
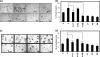
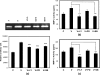
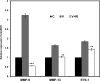
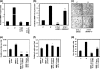
Matrix metalloproteinase-mediated degradation of extracellular matrix is a crucial event for invasion and metastasis of malignant cells. The expressions of matrix metalloproteinases (MMPs) are regulated by different cytokines and growth factors. VEGF, a potent angiogenic cytokine, induces invasion of ovarian cancer cells through activation of MMPs. Here, we demonstrate that invasion and scattering in SKOV-3 cells were induced by VEGF through the activation of p38 MAPK and PI3K/AKT pathways. VEGF induced the expression of MMP-2, MMP-9, and MMP-13 and hence regulated the metastasis of SKOV-3 ovarian cancer cells, and the activities of these MMPs were reduced after inhibition of PI3K/AKT and p38 MAPK pathways. Interestingly, VEGF induced expression of ETS-1 factor, an important trans-regulator of different MMP genes. ETS-1 bound to both MMP-9 and MMP-13 promoters. Furthermore, VEGF acted through its receptor to perform the said functions. In addition, VEGF-induced MMP-9 and MMP-13 expression and in vitro cell invasion were significantly reduced after knockdown of ETS-1 gene. Again, VEGF-induced MMP-9 and MMP-13 promoter activities were down-regulated in ETS-1 siRNA-transfected cells. VEGF enriched ETS-1 in the nuclear fraction in a dose-dependent manner. VEGF-induced expression of ETS-1 and its nuclear localization were blocked by specific inhibitors of the PI3K and p38 MAPK pathways. Therefore, based on these observations, it is hypothesized that the activation of PI3K/AKT and p38 MAPK by VEGF results in ETS-1 gene expression, which activates MMP-9 and MMP-13, leading to the invasion and scattering of SKOV-3 cells. The study provides a mechanistic insight into the prometastatic functions of VEGF-induced expression of relevant MMPs.
Figures




Similar articles
-
Blockade of Ets-1 attenuates epidermal growth factor-dependent collagen loss in human carotid plaque smooth muscle cells.Am J Physiol Heart Circ Physiol. 2015 Sep 15;309(6):H1075-86. doi: 10.1152/ajpheart.00378.2015. Epub 2015 Aug 7. Am J Physiol Heart Circ Physiol. 2015. PMID: 26254334 Free PMC article.
-
Visfatin induces human endothelial VEGF and MMP-2/9 production via MAPK and PI3K/Akt signalling pathways: novel insights into visfatin-induced angiogenesis.Cardiovasc Res. 2008 May 1;78(2):356-65. doi: 10.1093/cvr/cvm111. Epub 2007 Dec 18. Cardiovasc Res. 2008. PMID: 18093986
-
Sulfated polysaccharide of Sepiella Maindroni ink inhibits the migration, invasion and matrix metalloproteinase-2 expression through suppressing EGFR-mediated p38/MAPK and PI3K/Akt/mTOR signaling pathways in SKOV-3 cells.Int J Biol Macromol. 2018 Feb;107(Pt A):349-362. doi: 10.1016/j.ijbiomac.2017.08.178. Epub 2017 Sep 9. Int J Biol Macromol. 2018. PMID: 28870748
-
Metalloproteinases in Ovarian Cancer.Int J Mol Sci. 2021 Mar 26;22(7):3403. doi: 10.3390/ijms22073403. Int J Mol Sci. 2021. PMID: 33810259 Free PMC article. Review.
-
Phosphoinositide 3-kinase signalling pathways in tumor progression, invasion and angiogenesis.Tumori. 2004 Jan-Feb;90(1):2-8. doi: 10.1177/030089160409000102. Tumori. 2004. PMID: 15143962 Review.
Cited by
-
Combination of lapatinib with isothiocyanates overcomes drug resistance and inhibits migration of HER2 positive breast cancer cells.Breast Cancer. 2017 Mar;24(2):271-280. doi: 10.1007/s12282-016-0700-9. Epub 2016 May 6. Breast Cancer. 2017. PMID: 27154770 Free PMC article.
-
High expression level of MMP9 is associated with poor prognosis in patients with clear cell renal carcinoma.PeerJ. 2018 Jul 4;6:e5050. doi: 10.7717/peerj.5050. eCollection 2018. PeerJ. 2018. PMID: 30013825 Free PMC article.
-
Integrin αvβ6 and transcriptional factor Ets-1 act as prognostic indicators in colorectal cancer.Cell Biosci. 2014 Sep 2;4(1):53. doi: 10.1186/2045-3701-4-53. eCollection 2014. Cell Biosci. 2014. PMID: 25264483 Free PMC article.
-
Biosemantics guided gene expression profiling of Sjögren's syndrome: a comparative analysis with systemic lupus erythematosus and rheumatoid arthritis.Arthritis Res Ther. 2017 Aug 17;19(1):192. doi: 10.1186/s13075-017-1400-3. Arthritis Res Ther. 2017. PMID: 28818099 Free PMC article.
-
Plexiform Arteriopathy in Rodent Models of Pulmonary Arterial Hypertension.Am J Pathol. 2019 Jun;189(6):1133-1144. doi: 10.1016/j.ajpath.2019.02.005. Epub 2019 Mar 26. Am J Pathol. 2019. PMID: 30926336 Free PMC article. Review.
References
-
- Yap T. A., Carden C. P., Kaye S. B. (2009) Beyond chemotherapy: targeted therapies in ovarian cancer. Nat. Rev. Cancer 9, 167–181 - PubMed
-
- Bérubé M., Deschambeault A., Boucher M., Germain L., Petitclerc E., Guérin S. L. (2005) MMP-2 expression in uveal melanoma: differential activation status dictated by the cellular environment. Mol. Vis. 11, 1101–1111 - PubMed
-
- Sato T., Sakai T., Noguchi Y., Takita M., Hirakawa S., Ito A. (2004) Tumor-stromal cell contact promotes invasion of human uterine cervical carcinoma cells by augmenting the expression and activation of stromal matrix metalloproteinases. Gynecol. Oncol. 92, 47–56 - PubMed
-
- Di Nezza L. A., Misajon A., Zhang J., Jobling T., Quinn M. A., Ostör A. G., Nie G., Lopata A., Salamonsen L. A. (2002) Presence of active gelatinases in endometrial carcinoma and correlation of matrix metalloproteinase expression with increasing tumor grade and invasion. Cancer 94, 1466–1475 - PubMed
-
- Liotta L. A., Tryggvason K., Garbisa S., Hart I., Foltz C. M., Shafie S. (1980) Metastatic potential correlates with enzymatic degradation of basement membrane collagen. Nature 284, 67–68 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

