Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans
- PMID: 22269323
- PMCID: PMC3266793
- DOI: 10.1172/JCI60433
Brown adipose tissue oxidative metabolism contributes to energy expenditure during acute cold exposure in humans
Abstract
Brown adipose tissue (BAT) is vital for proper thermogenesis during cold exposure in rodents, but until recently its presence in adult humans and its contribution to human metabolism were thought to be minimal or insignificant. Recent studies using PET with 18F-fluorodeoxyglucose (18FDG) have shown the presence of BAT in adult humans. However, whether BAT contributes to cold-induced nonshivering thermogenesis in humans has not been proven. Using PET with 11C-acetate, 18FDG, and 18F-fluoro-thiaheptadecanoic acid (18FTHA), a fatty acid tracer, we have quantified BAT oxidative metabolism and glucose and nonesterified fatty acid (NEFA) turnover in 6 healthy men under controlled cold exposure conditions. All subjects displayed substantial NEFA and glucose uptake upon cold exposure. Furthermore, we demonstrated cold-induced activation of oxidative metabolism in BAT, but not in adjoining skeletal muscles and subcutaneous adipose tissue. This activation was associated with an increase in total energy expenditure. We found an inverse relationship between BAT activity and shivering. We also observed an increase in BAT radio density upon cold exposure, indicating reduced BAT triglyceride content. In sum, our study provides evidence that BAT acts as a nonshivering thermogenesis effector in humans.
Figures
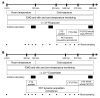


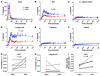
Comment in
-
Yes, even human brown fat is on fire!J Clin Invest. 2012 Feb;122(2):486-9. doi: 10.1172/JCI60941. Epub 2012 Jan 24. J Clin Invest. 2012. PMID: 22269320 Free PMC article.
Similar articles
-
In vivo measurement of energy substrate contribution to cold-induced brown adipose tissue thermogenesis.FASEB J. 2015 May;29(5):2046-58. doi: 10.1096/fj.14-266247. Epub 2015 Feb 13. FASEB J. 2015. PMID: 25681456
-
Selective Impairment of Glucose but Not Fatty Acid or Oxidative Metabolism in Brown Adipose Tissue of Subjects With Type 2 Diabetes.Diabetes. 2015 Jul;64(7):2388-97. doi: 10.2337/db14-1651. Epub 2015 Feb 12. Diabetes. 2015. PMID: 25677914
-
Cold-Induced Brown Adipose Tissue Activity Alters Plasma Fatty Acids and Improves Glucose Metabolism in Men.J Clin Endocrinol Metab. 2017 Nov 1;102(11):4226-4234. doi: 10.1210/jc.2017-01250. J Clin Endocrinol Metab. 2017. PMID: 28945846 Clinical Trial.
-
Brown adipose tissue and the regulation of nonshivering thermogenesis.Curr Opin Clin Nutr Metab Care. 2012 Nov;15(6):547-52. doi: 10.1097/MCO.0b013e3283599184. Curr Opin Clin Nutr Metab Care. 2012. PMID: 23037904 Review.
-
Substrate Utilization by Brown Adipose Tissue: What's Hot and What's Not?Front Endocrinol (Lausanne). 2020 Sep 25;11:571659. doi: 10.3389/fendo.2020.571659. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33101206 Free PMC article. Review.
Cited by
-
BMP7 drives human adipogenic stem cells into metabolically active beige adipocytes.Lipids. 2015 Feb;50(2):111-20. doi: 10.1007/s11745-014-3981-9. Epub 2014 Dec 23. Lipids. 2015. PMID: 25534037 Free PMC article.
-
Standardized In Vitro Models of Human Adipose Tissue Reveal Metabolic Flexibility in Brown Adipocyte Thermogenesis.Endocrinology. 2023 Nov 2;164(12):bqad161. doi: 10.1210/endocr/bqad161. Endocrinology. 2023. PMID: 37944134 Free PMC article.
-
Targeting adipose tissue.Diabetol Metab Syndr. 2012 Oct 27;4(1):43. doi: 10.1186/1758-5996-4-43. Diabetol Metab Syndr. 2012. PMID: 23102228 Free PMC article.
-
Systemic β-adrenergic stimulation of thermogenesis is not accompanied by brown adipose tissue activity in humans.Diabetes. 2012 Dec;61(12):3106-13. doi: 10.2337/db12-0288. Epub 2012 Aug 7. Diabetes. 2012. PMID: 22872233 Free PMC article.
-
Cold acclimation recruits human brown fat and increases nonshivering thermogenesis.J Clin Invest. 2013 Aug;123(8):3395-403. doi: 10.1172/JCI68993. Epub 2013 Jul 15. J Clin Invest. 2013. PMID: 23867626 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

