B-cell receptor: from resting state to activate
- PMID: 22269039
- PMCID: PMC3372753
- DOI: 10.1111/j.1365-2567.2012.03564.x
B-cell receptor: from resting state to activate
Abstract
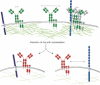
B-cell activation is triggered by the binding of antigen to the B-cell receptor (BCR). The early molecular events triggered by BCR binding of ligand have been well-characterized both biochemically and using optical microscopy techniques to visualize B-cell activation as it happens. However, we understand much less about the BCR before activation. For this reason, this review will address recent advances in our view of the structure, organization and dynamics of the resting, unstimulated BCR. These parameters have important implications for our understanding of the initiation of B-cell activation and will be discussed in the context of current models for BCR activation. These models include the conformation-induced oligomerization model, in which binding of antigen to monomeric BCR induces a pulling or twisting force causing conformational unmasking of a clustering interface in the Cμ4 domain. Conversely, the dissociation activation model proposes that BCRs exist in auto-inhibitory oligomers on the resting B-cell surface and binding of antigen promotes the dissociation of the BCR oligomer exposing phosphorylation residues within Igα/Igβ. Finally, the collision coupling model suggests that BCR are segregated from activating co-receptors or kinases and activation is associated with changes in BCR mobility on the cell surface, which allows for the functional interaction of these elements.
© 2012 The Author. Immunology © 2012 Blackwell Publishing Ltd.
Figures
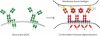

Similar articles
-
Receptor Dissociation and B-Cell Activation.Curr Top Microbiol Immunol. 2016;393:27-43. doi: 10.1007/82_2015_482. Curr Top Microbiol Immunol. 2016. PMID: 26428245 Review.
-
Oligomeric organization of the B-cell antigen receptor on resting cells.Nature. 2010 Sep 23;467(7314):465-9. doi: 10.1038/nature09357. Epub 2010 Sep 5. Nature. 2010. PMID: 20818374
-
Conformational change within the extracellular domain of B cell receptor in B cell activation upon antigen binding.Elife. 2019 Jul 10;8:e42271. doi: 10.7554/eLife.42271. Elife. 2019. PMID: 31290744 Free PMC article.
-
Basal Igalpha/Igbeta signals trigger the coordinated initiation of pre-B cell antigen receptor-dependent processes.J Immunol. 2004 Jul 15;173(2):1000-11. doi: 10.4049/jimmunol.173.2.1000. J Immunol. 2004. PMID: 15240688
-
Isotype Specific Assembly of B Cell Antigen Receptors and Synergism With Chemokine Receptor CXCR4.Front Immunol. 2018 Dec 18;9:2988. doi: 10.3389/fimmu.2018.02988. eCollection 2018. Front Immunol. 2018. PMID: 30619343 Free PMC article. Review.
Cited by
-
A computational model for early events in B cell antigen receptor signaling: analysis of the roles of Lyn and Fyn.J Immunol. 2012 Jul 15;189(2):646-58. doi: 10.4049/jimmunol.1102003. Epub 2012 Jun 18. J Immunol. 2012. PMID: 22711887 Free PMC article.
-
Enhanced oligomerization of full-length RAGE by synergy of the interaction of its domains.Sci Rep. 2019 Dec 30;9(1):20332. doi: 10.1038/s41598-019-56993-9. Sci Rep. 2019. PMID: 31889156 Free PMC article.
-
A Mini-Atlas of Gene Expression for the Domestic Goat (Capra hircus).Front Genet. 2019 Nov 4;10:1080. doi: 10.3389/fgene.2019.01080. eCollection 2019. Front Genet. 2019. PMID: 31749840 Free PMC article.
-
Immune Imprinting and Implications for COVID-19.Vaccines (Basel). 2023 Apr 20;11(4):875. doi: 10.3390/vaccines11040875. Vaccines (Basel). 2023. PMID: 37112787 Free PMC article. Review.
-
scRNA-seq analysis discovered suppression of immunomodulatory dependent inflammatory response in PMBCs exposed to silver nanoparticles.J Nanobiotechnology. 2024 Mar 17;22(1):118. doi: 10.1186/s12951-024-02364-0. J Nanobiotechnology. 2024. PMID: 38494495 Free PMC article.
References
-
- Lanzavecchia A. Antigen-specific interaction between T and B cells. Nature. 1985;314:537–9. - PubMed
-
- DeFranco AL. The complexity of signaling pathways activated by the BCR. Curr Opin Immunol. 1997;9:296–308. - PubMed
-
- Kurosaki T. Regulation of BCR signaling. Mol Immunol. 2011;48:1287–91. - PubMed
-
- Reth M, Wienands J. Initiation and processing of signals from the B cell antigen receptor. Annu Rev Immunol. 1997;15:453–79. - PubMed
-
- Harwood NE, Batista FD. New insights into the early molecular events underlying B cell activation. Immunity. 2008;28:609–19. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

