Novel myeloma-associated antigens revealed in the context of syngeneic hematopoietic stem cell transplantation
- PMID: 22267603
- PMCID: PMC3321874
- DOI: 10.1182/blood-2011-11-388926
Novel myeloma-associated antigens revealed in the context of syngeneic hematopoietic stem cell transplantation
Abstract
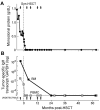
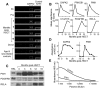
Targets of curative donor-derived graft-versus-myeloma (GVM) responses after allogeneic hematopoietic stem cell transplantation (HSCT) remain poorly defined, partly because immunity against minor histocompatibility Ags (mHAgs) complicates the elucidation of multiple myeloma (MM)-specific targets. We hypothesized that syngeneic HSCT would facilitate the identification of GVM-associated Ags because donor immune responses in this setting should exclusively target unique tumor Ags in the absence of donor-host genetic disparities. Therefore, in the present study, we investigated the development of tumor immunity in an HLA-A0201(+) MM patient who achieved durable remission after myeloablative syngeneic HSCT. Using high-density protein microarrays to screen post-HSCT plasma, we identified 6 Ags that elicited high-titer (1:5000-1:10 000) Abs that correlated with clinical tumor regression. Two Ags (DAPK2 and PIM1) had enriched expression in primary MM tissues. Both elicited Ab responses in other MM patients after chemotherapy or HSCT (11 and 6 of 32 patients for DAPK2 and PIM1, respectively). The index patient also developed specific CD8(+) T-cell responses to HLA-A2-restricted peptides derived from DAPK2 and PIM1. Peptide-specific T cells recognized HLA-A2(+) MM-derived cell lines and primary MM tumor cells. Coordinated T- and B-cell immunity develops against MM-associated Ags after syngeneic HSCT. DAPK1 and PIM1 are promising target Ags for MM-directed immunotherapy.
Figures


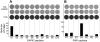


Similar articles
-
Towards effective and safe immunotherapy after allogeneic stem cell transplantation: identification of hematopoietic-specific minor histocompatibility antigen UTA2-1.Leukemia. 2013 Mar;27(3):642-9. doi: 10.1038/leu.2012.277. Epub 2012 Oct 1. Leukemia. 2013. PMID: 23079962 Free PMC article.
-
Allogeneic stem cell transplantation for multiple myeloma.Rev Clin Exp Hematol. 2002 Sep;6(3):205-24. doi: 10.1046/j.1468-0734.2002.00075.x. Rev Clin Exp Hematol. 2002. PMID: 12616696 Review.
-
Sperm protein 17 (Sp17) in multiple myeloma: opportunity for myeloma-specific donor T cell infusion to enhance graft-versus-myeloma effect without increasing graft-versus-host disease risk.Eur J Immunol. 2001 Aug;31(8):2277-83. doi: 10.1002/1521-4141(200108)31:8<2277::aid-immu2277>3.0.co;2-z. Eur J Immunol. 2001. PMID: 11477539
-
Establishment of a murine graft-versus-myeloma model using allogeneic stem cell transplantation.PLoS One. 2014 Nov 21;9(11):e113764. doi: 10.1371/journal.pone.0113764. eCollection 2014. PLoS One. 2014. PMID: 25415267 Free PMC article.
-
Non-myeloablative hematopoietic stem cell transplantation (NST) in the treatment of human malignancies: from animal models to clinical practice.Cancer Treat Res. 2002;110:113-36. doi: 10.1007/978-1-4615-0919-6_6. Cancer Treat Res. 2002. PMID: 11908195 Review.
Cited by
-
T cell optimization for graft-versus-leukemia responses.JCI Insight. 2020 May 7;5(9):e134939. doi: 10.1172/jci.insight.134939. JCI Insight. 2020. PMID: 32376800 Free PMC article. Review.
-
Autologous CLL cell vaccination early after transplant induces leukemia-specific T cells.J Clin Invest. 2013 Sep;123(9):3756-65. doi: 10.1172/JCI69098. Epub 2013 Aug 5. J Clin Invest. 2013. PMID: 23912587 Free PMC article. Clinical Trial.
-
Development of a coordinated allo T cell and auto B cell response against autosomal PTK2B after allogeneic hematopoietic stem cell transplantation.Haematologica. 2014 Feb;99(2):365-9. doi: 10.3324/haematol.2013.086652. Epub 2013 Oct 4. Haematologica. 2014. PMID: 24097630 Free PMC article. Clinical Trial.
-
Peptide vaccines for hematological malignancies: a missed promise?Int J Hematol. 2014 Feb;99(2):107-16. doi: 10.1007/s12185-013-1497-3. Epub 2014 Jan 8. Int J Hematol. 2014. PMID: 24399190 Review.
-
Antigen Discovery and Therapeutic Targeting in Hematologic Malignancies.Cancer J. 2017 Mar/Apr;23(2):115-124. doi: 10.1097/PPO.0000000000000257. Cancer J. 2017. PMID: 28410299 Free PMC article. Review.
References
-
- Horowitz MM, Gale RP, Sondel PM, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75(3):555–562. - PubMed
-
- Alyea EP, Soiffer RJ, Canning C, et al. Toxicity and efficacy of defined doses of CD4(+) donor lymphocytes for treatment of relapse after allogeneic bone marrow transplant. Blood. 1998;91(10):3671–3680. - PubMed
-
- Lokhorst HM, Schattenberg A, Cornelissen JJ, Thomas LL, Verdonck LF. Donor leukocyte infusions are effective in relapsed multiple myeloma after allogeneic bone marrow transplantation. Blood. 1997;90(10):4206–4211. - PubMed
-
- Crawley C, Lalancette M, Szydlo R, et al. Outcomes for reduced-intensity allogeneic transplantation for multiple myeloma: an analysis of prognostic factors from the Chronic Leukaemia Working Party of the EBMT. Blood. 2005;105(11):4532–4539. - PubMed
-
- Maloney DG, Molina AJ, Sahebi F, et al. Allografting with nonmyeloablative conditioning following cytoreductive autografts for the treatment of patients with multiple myeloma. Blood. 2003;102(9):3447–3454. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

