Structure and activation of rhodopsin
- PMID: 22266727
- PMCID: PMC3677203
- DOI: 10.1038/aps.2011.171
Structure and activation of rhodopsin
Abstract
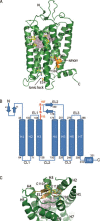
Rhodopsin is the first G-protein-coupled receptor (GPCR) with its three-dimensional structure solved by X-ray crystallography. The crystal structure of rhodopsin has revealed the molecular mechanism of photoreception and signal transduction in the visual system. Although several other GPCR crystal structures have been reported over the past few years, the rhodopsin structure remains an important model for understanding the structural and functional characteristics of other GPCRs. This review summarizes the structural features, the photoactivation, and the G protein signal transduction of rhodopsin.
Figures

Similar articles
-
Comparative sequence and structural analyses of G-protein-coupled receptor crystal structures and implications for molecular models.PLoS One. 2009 Sep 16;4(9):e7011. doi: 10.1371/journal.pone.0007011. PLoS One. 2009. PMID: 19756152 Free PMC article.
-
Crystal structure of rhodopsin in complex with a mini-Go sheds light on the principles of G protein selectivity.Sci Adv. 2018 Sep 19;4(9):eaat7052. doi: 10.1126/sciadv.aat7052. eCollection 2018 Sep. Sci Adv. 2018. PMID: 30255144 Free PMC article.
-
Relevance of rhodopsin studies for GPCR activation.Biochim Biophys Acta. 2014 May;1837(5):674-82. doi: 10.1016/j.bbabio.2013.09.002. Epub 2013 Sep 13. Biochim Biophys Acta. 2014. PMID: 24041646 Review.
-
Activation of G-protein-coupled receptors: a common molecular mechanism.Trends Endocrinol Metab. 2003 Nov;14(9):431-7. doi: 10.1016/j.tem.2003.09.007. Trends Endocrinol Metab. 2003. PMID: 14580763 Review.
-
Restructuring G-protein- coupled receptor activation.Cell. 2012 Sep 28;151(1):14-23. doi: 10.1016/j.cell.2012.09.003. Cell. 2012. PMID: 23021212 Review.
Cited by
-
Characterizing variants of unknown significance in rhodopsin: A functional genomics approach.Hum Mutat. 2019 Aug;40(8):1127-1144. doi: 10.1002/humu.23762. Epub 2019 Jun 22. Hum Mutat. 2019. PMID: 30977563 Free PMC article.
-
Orphan G protein receptor GPR55 as an emerging target in cancer therapy and management.Cancer Manag Res. 2013 Jul 1;5:147-55. doi: 10.2147/CMAR.S35175. Print 2013. Cancer Manag Res. 2013. PMID: 23869178 Free PMC article.
-
Systematic Assessment of Protein C-Termini Mutated in Human Disorders.Biomolecules. 2023 Feb 12;13(2):355. doi: 10.3390/biom13020355. Biomolecules. 2023. PMID: 36830724 Free PMC article.
-
Advances in methods to characterize ligand-induced ionic lock and rotamer toggle molecular switch in G protein-coupled receptors.Methods Enzymol. 2013;520:153-74. doi: 10.1016/B978-0-12-391861-1.00007-1. Methods Enzymol. 2013. PMID: 23332699 Free PMC article.
-
iTRAQ Quantitative Proteomic Analysis of Vitreous from Patients with Retinal Detachment.Int J Mol Sci. 2018 Apr 11;19(4):1157. doi: 10.3390/ijms19041157. Int J Mol Sci. 2018. PMID: 29641463 Free PMC article.
References
-
- Fredriksson R, Lagerström MC, Lundin LG, Schiöth HB. The G-protein-coupled receptors in the human genome form five main families. Phylogenetic analysis, paralogon groups, and fingerprints. Mol Pharmacol. 2003;63:17. - PubMed
-
- Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, et al. Crystal structure of rhodopsin: A G protein-coupled receptor. Science. 2000;289:739–45. - PubMed
-
- Li J, Edwards PC, Burghammer M, Villa C, Schertler GF. Structure of bovine rhodopsin in a trigonal crystal form. J Mol Biol. 2004;343:1409–38. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

