Peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) enhances engraftment and angiogenesis of mesenchymal stem cells in diabetic hindlimb ischemia
- PMID: 22266669
- PMCID: PMC3331776
- DOI: 10.2337/db11-1271
Peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α) enhances engraftment and angiogenesis of mesenchymal stem cells in diabetic hindlimb ischemia
Abstract
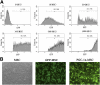
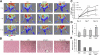
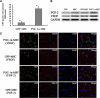
To examine whether the peroxisome proliferator-activated receptor-γ coactivator-1α (PGC-1α), a key regulator linking angiogenesis and metabolism, could enhance the engraftment and angiogenesis of mesenchymal stem cells (MSCs) in diabetic hindlimb ischemia, we engineered the overexpression of PGC-1α within MSCs using an adenoviral vector encoding green fluorescent protein and PGC-1α, and then tested the survivability and angiogenesis of MSCs in vitro and in vivo. Under the condition of hypoxia concomitant with serum deprivation, the overexpression of PGC-1α in MSCs resulted in a higher expression level of hypoxia-inducible factor-1α (Hif-1α), a greater ratio of B-cell lymphoma leukemia-2 (Bcl-2)/Bcl-2-associated X protein (Bax), and a lower level of caspase 3 compared with the controls, followed by an increased survival rate and an elevated expression level of several proangiogenic factors. In vivo, the MSCs modified with PGC-1α could significantly increase the blood perfusion and capillary density of ischemic hindlimb of the diabetic rats, which was correlated to an improved survivability of MSCs and an increased level of several proangiogenic factors secreted by MSCs. We identified for the first time that PGC-1α could enhance the engraftment and angiogenesis of MSCs in diabetic hindlimb ischemia.
Figures


Comment in
-
PGC-1α: the missing ingredient for mesenchymal stem cell-mediated angiogenesis.Diabetes. 2012 May;61(5):979-80. doi: 10.2337/db12-0078. Diabetes. 2012. PMID: 22517649 Free PMC article. No abstract available.
Similar articles
-
PGC-1α: the missing ingredient for mesenchymal stem cell-mediated angiogenesis.Diabetes. 2012 May;61(5):979-80. doi: 10.2337/db12-0078. Diabetes. 2012. PMID: 22517649 Free PMC article. No abstract available.
-
PGC-1α induces SPP1 to activate macrophages and orchestrate functional angiogenesis in skeletal muscle.Circ Res. 2014 Aug 15;115(5):504-17. doi: 10.1161/CIRCRESAHA.115.303829. Epub 2014 Jul 9. Circ Res. 2014. PMID: 25009290 Free PMC article.
-
Hyperhomocysteinemia attenuates angiogenesis through reduction of HIF-1α and PGC-1α levels in muscle fibers during hindlimb ischemia.Am J Physiol Heart Circ Physiol. 2014 Apr 15;306(8):H1116-27. doi: 10.1152/ajpheart.00003.2014. Epub 2014 Feb 28. Am J Physiol Heart Circ Physiol. 2014. PMID: 24585779 Free PMC article.
-
Mesenchymal stem cells as a treatment for peripheral arterial disease: current status and potential impact of type II diabetes on their therapeutic efficacy.Stem Cell Rev Rep. 2013 Jun;9(3):360-72. doi: 10.1007/s12015-013-9433-8. Stem Cell Rev Rep. 2013. PMID: 23475434 Free PMC article. Review.
-
Therapeutic Applications of Engineered Mesenchymal Stromal Cells for Enhanced Angiogenesis in Cardiac and Cerebral Ischemia.Stem Cell Rev Rep. 2024 Nov;20(8):2138-2154. doi: 10.1007/s12015-024-10787-3. Epub 2024 Sep 21. Stem Cell Rev Rep. 2024. PMID: 39305405 Free PMC article. Review.
Cited by
-
The potential of cell-based therapy for diabetes and diabetes-related vascular complications.Curr Diab Rep. 2014 Mar;14(3):469. doi: 10.1007/s11892-013-0469-6. Curr Diab Rep. 2014. PMID: 24464340 Review.
-
Systematic review and meta-analysis of the effect of bone marrow-derived cell therapies on hind limb perfusion.Dis Model Mech. 2024 May 1;17(5):dmm050632. doi: 10.1242/dmm.050632. Epub 2024 May 24. Dis Model Mech. 2024. PMID: 38616715 Free PMC article.
-
Implantation of Hypoxia-Induced Mesenchymal Stem Cell Advances Therapeutic Angiogenesis.Stem Cells Int. 2022 Mar 20;2022:6795274. doi: 10.1155/2022/6795274. eCollection 2022. Stem Cells Int. 2022. PMID: 35355589 Free PMC article.
-
Natural products, PGC-1 α , and Duchenne muscular dystrophy.Acta Pharm Sin B. 2020 May;10(5):734-745. doi: 10.1016/j.apsb.2020.01.001. Epub 2020 Jan 8. Acta Pharm Sin B. 2020. PMID: 32528825 Free PMC article. Review.
-
Lysozyme-Antimicrobial Peptide Fusion Protein Promotes the Diabetic Wound Size Reduction in Streptozotocin (STZ)-Induced Diabetic Rats.Med Sci Monit. 2018 Nov 23;24:8449-8458. doi: 10.12659/MSM.912596. Med Sci Monit. 2018. PMID: 30468157 Free PMC article.
References
-
- Newton KM, Wagner EH, Ramsey SD, et al. The use of automated data to identify complications and comorbidities of diabetes: a validation study. J Clin Epidemiol 1999;52:199–207 - PubMed
-
- O’Loughlin A, McIntosh C, Dinneen SF, O’Brien T. Review paper: basic concepts to novel therapies: a review of the diabetic foot. Int J Low Extrem Wounds 2010;9:90–102 - PubMed
-
- Kamihata H, Matsubara H, Nishiue T, et al. Implantation of bone marrow mononuclear cells into ischemic myocardium enhances collateral perfusion and regional function via side supply of angioblasts, angiogenic ligands, and cytokines. Circulation 2001;104:1046–1052 - PubMed
-
- Johannesson A, Larsson GU, Ramstrand N, Turkiewicz A, Wiréhn AB, Atroshi I. Incidence of lower-limb amputation in the diabetic and nondiabetic general population: a 10-year population-based cohort study of initial unilateral and contralateral amputations and reamputations. Diabetes Care 2009;32:275–280 - PMC - PubMed
-
- Uccelli A, Moretta L, Pistoia V. Mesenchymal stem cells in health and disease. Nat Rev Immunol 2008;8:726–736 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

