Nitrogen monoxide (NO) storage and transport by dinitrosyl-dithiol-iron complexes: long-lived NO that is trafficked by interacting proteins
- PMID: 22262835
- PMCID: PMC3293582
- DOI: 10.1074/jbc.R111.329847
Nitrogen monoxide (NO) storage and transport by dinitrosyl-dithiol-iron complexes: long-lived NO that is trafficked by interacting proteins
Abstract
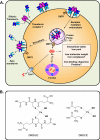
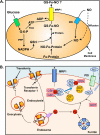
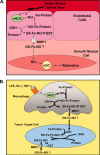
Nitrogen monoxide (NO) markedly affects intracellular iron metabolism, and recent studies have shown that molecules traditionally involved in drug resistance, namely GST and MRP1 (multidrug resistance-associated protein 1), are critical molecular players in this process. This is mediated by interaction of these proteins with dinitrosyl-dithiol-iron complexes (Watts, R. N., Hawkins, C., Ponka, P., and Richardson, D. R. (2006) Proc. Natl. Acad. Sci. U.S.A. 103, 7670-7675; Lok, H. C., Suryo Rahmanto, Y., Hawkins, C. L., Kalinowski, D. S., Morrow, C. S., Townsend, A. J., Ponka, P., and Richardson, D. R. (2012) J. Biol. Chem. 287, 607-618). These complexes are bioavailable, have a markedly longer half-life compared with free NO, and form in cells after an interaction between iron, NO, and glutathione. The generation of dinitrosyl-dithiol-iron complexes acts as a common currency for NO transport and storage by MRP1 and GST P1-1, respectively. Understanding the biological trafficking mechanisms involved in the metabolism of NO is vital for elucidating its many roles in cellular signaling and cytotoxicity and for development of new therapeutic targets.
Figures
Similar articles
-
Nitric oxide storage and transport in cells are mediated by glutathione S-transferase P1-1 and multidrug resistance protein 1 via dinitrosyl iron complexes.J Biol Chem. 2012 Jan 2;287(1):607-618. doi: 10.1074/jbc.M111.310987. Epub 2011 Nov 14. J Biol Chem. 2012. PMID: 22084240 Free PMC article.
-
Glutathione S-transferase and MRP1 form an integrated system involved in the storage and transport of dinitrosyl-dithiolato iron complexes in cells.Free Radic Biol Med. 2014 Oct;75:14-29. doi: 10.1016/j.freeradbiomed.2014.07.002. Epub 2014 Jul 15. Free Radic Biol Med. 2014. PMID: 25035074 Review.
-
The Relationship of Glutathione-S-Transferase and Multi-Drug Resistance-Related Protein 1 in Nitric Oxide (NO) Transport and Storage.Molecules. 2021 Sep 24;26(19):5784. doi: 10.3390/molecules26195784. Molecules. 2021. PMID: 34641326 Free PMC article. Review.
-
Regulation and control of nitric oxide (NO) in macrophages: Protecting the "professional killer cell" from its own cytotoxic arsenal via MRP1 and GSTP1.Biochim Biophys Acta Gen Subj. 2017 May;1861(5 Pt A):995-999. doi: 10.1016/j.bbagen.2017.02.021. Epub 2017 Feb 17. Biochim Biophys Acta Gen Subj. 2017. PMID: 28219722 Review.
-
Glutathione transferases sequester toxic dinitrosyl-iron complexes in cells. A protection mechanism against excess nitric oxide.J Biol Chem. 2007 Mar 2;282(9):6364-71. doi: 10.1074/jbc.M609905200. Epub 2006 Dec 30. J Biol Chem. 2007. PMID: 17197702
Cited by
-
Cold Argon Athmospheric Plasma for Biomedicine: Biological Effects, Applications and Possibilities.Antioxidants (Basel). 2022 Jun 27;11(7):1262. doi: 10.3390/antiox11071262. Antioxidants (Basel). 2022. PMID: 35883753 Free PMC article. Review.
-
Nitrite potentiates the vasodilatory signaling of S-nitrosothiols.Nitric Oxide. 2018 May 1;75:60-69. doi: 10.1016/j.niox.2018.01.011. Epub 2018 Feb 8. Nitric Oxide. 2018. PMID: 29428841 Free PMC article.
-
Mitochondria of a human multidrug-resistant hepatocellular carcinoma cell line constitutively express inducible nitric oxide synthase in the inner membrane.J Cell Mol Med. 2015 Jun;19(6):1410-7. doi: 10.1111/jcmm.12528. Epub 2015 Feb 18. J Cell Mol Med. 2015. PMID: 25691007 Free PMC article.
-
A Shoot Fe Signaling Pathway Requiring the OPT3 Transporter Controls GSNO Reductase and Ethylene in Arabidopsis thaliana Roots.Front Plant Sci. 2018 Sep 11;9:1325. doi: 10.3389/fpls.2018.01325. eCollection 2018. Front Plant Sci. 2018. PMID: 30254659 Free PMC article.
-
The good Samaritan glutathione-S-transferase P1: An evolving relationship in nitric oxide metabolism mediated by the direct interactions between multiple effector molecules.Redox Biol. 2023 Feb;59:102568. doi: 10.1016/j.redox.2022.102568. Epub 2022 Dec 15. Redox Biol. 2023. PMID: 36563536 Free PMC article. Review.
References
-
- Stamler J. S., Singel D. J., Loscalzo J. (1992) Biochemistry of nitric oxide and its redox-activated forms. Science 258, 1898–1902 - PubMed
-
- Richardson D. R., Ponka P. (1997) The molecular mechanisms of the metabolism and transport of iron in normal and neoplastic cells. Biochim. Biophys. Acta 1331, 1–40 - PubMed
-
- Hibbs J. B., Jr., Taintor R. R., Vavrin Z. (1984) Iron depletion: possible cause of tumor cell cytotoxicity induced by activated macrophages. Biochem. Biophys. Res. Commun. 123, 716–723 - PubMed
-
- Henry Y., Ducrocq C., Drapier J. C., Servent D., Pellat C., Guissani A. (1991) Nitric oxide, a biological effector. Electron paramagnetic resonance detection of nitrosyl-iron-protein complexes in whole cells. Eur. Biophys. J. 20, 1–15 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

