Expression of long-form N-acetylglucosamine-6-O-sulfotransferase 1 in human high endothelial venules
- PMID: 22260995
- PMCID: PMC3351234
- DOI: 10.1369/0022155412437613
Expression of long-form N-acetylglucosamine-6-O-sulfotransferase 1 in human high endothelial venules
Abstract
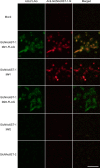
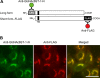
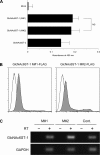
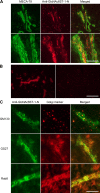
Two members of the N-acetylglucosamine-6-O-sulfotransferase (GlcNAc6ST) family, GlcNAc6ST-1 and GlcNAc6ST-2, function in the biosynthesis of 6-sulfo sialyl Lewis X-capped glycoproteins expressed on high endothelial venules (HEVs) in secondary lymphoid organs. Thus, both enzymes play a critical role in L-selectin-expressing lymphocyte homing. Human GlcNAc6ST-1 is encoded by a 1593-bp open reading frame exhibiting two 5' in-frame methionine codons spaced 141 bp apart. Both resemble the consensus sequence for translation initiation. Thus, it has been hypothesized that both long and short forms of GlcNAc6ST-1 may be present, although endogenous expression of either form has not been confirmed in humans. Here, the authors developed an antibody recognizing amino acid residues between the first two human GlcNAc6ST-1 methionines. This antibody specifically recognizes the long form of the enzyme, a finding validated by Western blot analysis and immunofluorescence cytochemistry of HeLa cells misexpressing long and/or short forms of human GlcNAc6ST-1. Using this antibody, the authors carried out immunofluorescence histochemistry of human lymph node tissue sections and found endogenous expression of the long form of the enzyme in human tissue, predominantly in the trans-Golgi network of endothelial cells that form HEVs.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures


Similar articles
-
N-acetylglucosamine 6-O-sulfotransferase-1 regulates expression of L-selectin ligands and lymphocyte homing.J Biol Chem. 2004 Aug 13;279(33):35001-8. doi: 10.1074/jbc.M404456200. Epub 2004 Jun 2. J Biol Chem. 2004. PMID: 15175329
-
Expression of N-acetylglucosamine 6-O-sulfotransferases (GlcNAc6STs)-1 and -4 in human monocytes: GlcNAc6ST-1 is implicated in the generation of the 6-sulfo N-acetyllactosamine/Lewis x epitope on CD44 and is induced by TNF-alpha.Glycobiology. 2005 Jul;15(7):7C-13C. doi: 10.1093/glycob/cwi050. Epub 2005 Feb 23. Glycobiology. 2005. PMID: 15728736
-
Detection of a sulfotransferase (HEC-GlcNAc6ST) in high endothelial venules of lymph nodes and in high endothelial venule-like vessels within ectopic lymphoid aggregates: relationship to the MECA-79 epitope.Am J Pathol. 2004 May;164(5):1635-44. doi: 10.1016/S0002-9440(10)63722-4. Am J Pathol. 2004. PMID: 15111310 Free PMC article.
-
Sulfated L-selectin ligands as a therapeutic target in chronic inflammation.Trends Immunol. 2006 Dec;27(12):559-65. doi: 10.1016/j.it.2006.10.007. Epub 2006 Oct 17. Trends Immunol. 2006. PMID: 17049924 Review.
-
Roles of sulfated glycans in lymphocyte homing.Biol Pharm Bull. 2006 Dec;29(12):2343-9. doi: 10.1248/bpb.29.2343. Biol Pharm Bull. 2006. PMID: 17142960 Review.
Cited by
-
Apical membrane expression of distinct sulfated glycans represents a novel marker of cholangiolocellular carcinoma.Lab Invest. 2016 Dec;96(12):1246-1255. doi: 10.1038/labinvest.2016.104. Epub 2016 Oct 17. Lab Invest. 2016. PMID: 27748735
-
Apical Membrane Expression of Distinct Sulfated Glycans Is a Characteristic Feature of Ductules and Their Reactive and Neoplastic Counterparts.J Histochem Cytochem. 2021 Sep;69(9):555-573. doi: 10.1369/00221554211035730. Epub 2021 Jul 30. J Histochem Cytochem. 2021. PMID: 34328046 Free PMC article.
-
The Conspicuousness of High Endothelial Venules in Angioimmunoblastic T-cell Lymphoma Is Due to Increased Cross-sectional Area, Not Increased Distribution Density.J Histochem Cytochem. 2021 Oct;69(10):645-657. doi: 10.1369/00221554211048551. J Histochem Cytochem. 2021. PMID: 34617807 Free PMC article.
-
Expression of Podocalyxin Potentially Decorated With Low-sulfated Keratan Sulfate in Human Testicular Embryonal Carcinoma.J Histochem Cytochem. 2024 Jul;72(7):453-465. doi: 10.1369/00221554241265162. Epub 2024 Jul 25. J Histochem Cytochem. 2024. PMID: 39051568
-
Paradoxical Expression of R-10G-reactive Antigen in Human Testicular Embryonal Carcinoma.J Histochem Cytochem. 2023 Oct;71(10):555-563. doi: 10.1369/00221554231199134. Epub 2023 Sep 7. J Histochem Cytochem. 2023. PMID: 37675782 Free PMC article.
References
-
- Aloisi F, Pujol-Borrell R. 2006. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 6:205–217 - PubMed
-
- Bhakta S, Bartes A, Bowman KG, Kao WM, Polsky I, Lee JK, Cook BN, Bruehl RE, Rosen SD, Bertozzi CR, et al. 2000. Sulfation of N-acetylglucosamine by chondroitin 6-sulfotransferase 2 (GST-5). J Biol Chem. 275:40226–40234 - PubMed
-
- Butcher EC, Picker LJ. 1996. Lymphocyte homing and homeostasis. Science. 272:60–66 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

