Mutation in SUMO E3 ligase, SIZ1, disrupts the mature female gametophyte in Arabidopsis
- PMID: 22253727
- PMCID: PMC3253799
- DOI: 10.1371/journal.pone.0029470
Mutation in SUMO E3 ligase, SIZ1, disrupts the mature female gametophyte in Arabidopsis
Abstract
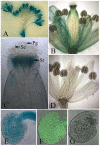
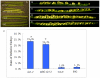
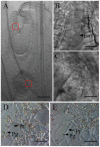
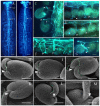
Female gametophyte is the multicellular haploid structure that can produce embryo and endosperm after fertilization, which has become an attractive model system for investigating molecular mechanisms in nuclei migration, cell specification, cell-to-cell communication and many other processes. Previous reports found that the small ubiquitin-like modifier (SUMO) E3 ligase, SIZ1, participated in many processes depending on particular target substrates and suppression of salicylic acid (SA) accumulation. Here, we report that SIZ1 mediates the reproductive process. SIZ1 showed enhanced expression in female organs, but was not detected in the anther or pollen. A defect in the siz1-2 maternal source resulted in reduced seed-set regardless of high SA concentration within the plant. Moreover, aniline blue staining and scanning electron microscopy revealed that funicular and micropylar pollen tube guidance was arrested in siz1-2 plants. Some of the embryo sacs of ovules in siz1-2 were also disrupted quickly after stage FG7. There was no significant affects of the siz1-2 mutation on expression of genes involved in female gametophyte development- or pollen tube guidance in ovaries. Together, our results suggest that SIZ1 sustains the stability and normal function of the mature female gametophyte which is necessary for pollen tube guidance.
Conflict of interest statement
Figures

Similar articles
-
An Arabidopsis SUMO E3 Ligase, SIZ1, Negatively Regulates Photomorphogenesis by Promoting COP1 Activity.PLoS Genet. 2016 Apr 29;12(4):e1006016. doi: 10.1371/journal.pgen.1006016. eCollection 2016 Apr. PLoS Genet. 2016. PMID: 27128446 Free PMC article.
-
Arabidopsis SUMO E3 ligase SIZ1 is involved in excess copper tolerance.Plant Physiol. 2011 Aug;156(4):2225-34. doi: 10.1104/pp.111.178996. Epub 2011 Jun 1. Plant Physiol. 2011. PMID: 21632972 Free PMC article.
-
SIZ1 small ubiquitin-like modifier E3 ligase facilitates basal thermotolerance in Arabidopsis independent of salicylic acid.Plant Physiol. 2006 Dec;142(4):1548-58. doi: 10.1104/pp.106.088831. Epub 2006 Oct 13. Plant Physiol. 2006. PMID: 17041025 Free PMC article.
-
How many receptor-like kinases are required to operate a pollen tube.Curr Opin Plant Biol. 2018 Feb;41:73-82. doi: 10.1016/j.pbi.2017.09.008. Epub 2017 Oct 6. Curr Opin Plant Biol. 2018. PMID: 28992536 Review.
-
Epigenetic control of cell specification during female gametogenesis.Sex Plant Reprod. 2011 Jun;24(2):137-47. doi: 10.1007/s00497-011-0166-z. Epub 2011 Apr 12. Sex Plant Reprod. 2011. PMID: 21484604 Review.
Cited by
-
Proteolysis in Reproduction: Lessons From Gene-Modified Organism Studies.Front Endocrinol (Lausanne). 2022 May 4;13:876370. doi: 10.3389/fendo.2022.876370. eCollection 2022. Front Endocrinol (Lausanne). 2022. PMID: 35600599 Free PMC article. Review.
-
The Maize Class-I SUMO Conjugating Enzyme ZmSCE1d Is Involved in Drought Stress Response.Int J Mol Sci. 2019 Dec 19;21(1):29. doi: 10.3390/ijms21010029. Int J Mol Sci. 2019. PMID: 31861556 Free PMC article.
-
Cold signaling and cold response in plants.Int J Mol Sci. 2013 Mar 6;14(3):5312-37. doi: 10.3390/ijms14035312. Int J Mol Sci. 2013. PMID: 23466881 Free PMC article.
-
The rice wall-associated receptor-like kinase gene OsDEES1 plays a role in female gametophyte development.Plant Physiol. 2012 Oct;160(2):696-707. doi: 10.1104/pp.112.203943. Epub 2012 Aug 10. Plant Physiol. 2012. PMID: 22885936 Free PMC article.
-
Two SUMO Proteases SUMO PROTEASE RELATED TO FERTILITY1 and 2 Are Required for Fertility in Arabidopsis.Plant Physiol. 2017 Dec;175(4):1703-1719. doi: 10.1104/pp.17.00021. Epub 2017 Oct 24. Plant Physiol. 2017. PMID: 29066667 Free PMC article.
References
-
- Higashiyama T, Yabe S, Sasaki N, Nishimura Y, Miyagishima S, et al. Pollen tube attraction by the synergid cell. Science. 2001;293:1480–1483. - PubMed
-
- Higashiyama T, Kuroiwa H, Kuroiwa T. Pollen-tube guidance: beacons from the female gametophyte. Curr Opin Plant Biol. 2003;6:36–41. - PubMed
-
- Okuda S, Tsutsui H, Shiina K, Sprunck S, Takeuchi H, et al. Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature. 2009;458:357–361. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

