A modular library of small molecule signals regulates social behaviors in Caenorhabditis elegans
- PMID: 22253572
- PMCID: PMC3254649
- DOI: 10.1371/journal.pbio.1001237
A modular library of small molecule signals regulates social behaviors in Caenorhabditis elegans
Abstract
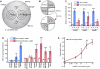
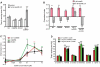
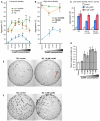
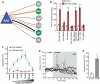
The nematode C. elegans is an important model for the study of social behaviors. Recent investigations have shown that a family of small molecule signals, the ascarosides, controls population density sensing and mating behavior. However, despite extensive studies of C. elegans aggregation behaviors, no intraspecific signals promoting attraction or aggregation of wild-type hermaphrodites have been identified. Using comparative metabolomics, we show that the known ascarosides are accompanied by a series of derivatives featuring a tryptophan-derived indole moiety. Behavioral assays demonstrate that these indole ascarosides serve as potent intraspecific attraction and aggregation signals for hermaphrodites, in contrast to ascarosides lacking the indole group, which are repulsive. Hermaphrodite attraction to indole ascarosides depends on the ASK amphid sensory neurons. Downstream of the ASK sensory neuron, the interneuron AIA is required for mediating attraction to indole ascarosides instead of the RMG interneurons, which previous studies have shown to integrate attraction and aggregation signals from ASK and other sensory neurons. The role of the RMG interneuron in mediating aggregation and attraction is thought to depend on the neuropeptide Y-like receptor NPR-1, because solitary and social C. elegans strains are distinguished by different npr-1 variants. We show that indole ascarosides promote attraction and aggregation in both solitary and social C. elegans strains. The identification of indole ascarosides as aggregation signals reveals unexpected complexity of social signaling in C. elegans, which appears to be based on a modular library of ascarosides integrating building blocks derived from lipid β-oxidation and amino-acid metabolism. Variation of modules results in strongly altered signaling content, as addition of a tryptophan-derived indole unit to repellent ascarosides produces strongly attractive indole ascarosides. Our findings show that the library of ascarosides represents a highly developed chemical language integrating different neurophysiological pathways to mediate social communication in C. elegans.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Comment in
-
New signaling chemicals spur worms to seek company.PLoS Biol. 2012 Jan;10(1):e1001240. doi: 10.1371/journal.pbio.1001240. Epub 2012 Jan 10. PLoS Biol. 2012. PMID: 22253575 Free PMC article. No abstract available.
Similar articles
-
A blend of small molecules regulates both mating and development in Caenorhabditis elegans.Nature. 2008 Aug 28;454(7208):1115-8. doi: 10.1038/nature07168. Epub 2008 Jul 23. Nature. 2008. PMID: 18650807 Free PMC article.
-
A hub-and-spoke circuit drives pheromone attraction and social behaviour in C. elegans.Nature. 2009 Apr 30;458(7242):1171-5. doi: 10.1038/nature07886. Epub 2009 Apr 6. Nature. 2009. PMID: 19349961 Free PMC article.
-
Comparative metabolomics reveals biogenesis of ascarosides, a modular library of small-molecule signals in C. elegans.J Am Chem Soc. 2012 Jan 25;134(3):1817-24. doi: 10.1021/ja210202y. Epub 2012 Jan 12. J Am Chem Soc. 2012. PMID: 22239548 Free PMC article.
-
Ascaroside signaling in C. elegans.WormBook. 2013 Jan 18:1-22. doi: 10.1895/wormbook.1.155.1. WormBook. 2013. PMID: 23355522 Free PMC article. Review.
-
Chemical mating cues in C. elegans.Semin Cell Dev Biol. 2014 Sep;33:18-24. doi: 10.1016/j.semcdb.2014.06.002. Epub 2014 Jun 27. Semin Cell Dev Biol. 2014. PMID: 24977334 Review.
Cited by
-
Sex-specific mating pheromones in the nematode Panagrellus redivivus.Proc Natl Acad Sci U S A. 2012 Dec 18;109(51):20949-54. doi: 10.1073/pnas.1218302109. Epub 2012 Dec 3. Proc Natl Acad Sci U S A. 2012. PMID: 23213209 Free PMC article.
-
Ascaroside signaling is widely conserved among nematodes.Curr Biol. 2012 May 8;22(9):772-80. doi: 10.1016/j.cub.2012.03.024. Epub 2012 Apr 12. Curr Biol. 2012. PMID: 22503501 Free PMC article.
-
The mutational structure of metabolism in Caenorhabditis elegans.Evolution. 2016 Oct;70(10):2239-2246. doi: 10.1111/evo.13020. Epub 2016 Aug 24. Evolution. 2016. PMID: 27465022 Free PMC article.
-
Photoaffinity probes for nematode pheromone receptor identification.Org Biomol Chem. 2019 Dec 18;18(1):36-40. doi: 10.1039/c9ob02099c. Org Biomol Chem. 2019. PMID: 31781713 Free PMC article.
-
Intestinal peroxisomal fatty acid β-oxidation regulates neural serotonin signaling through a feedback mechanism.PLoS Biol. 2019 Dec 5;17(12):e3000242. doi: 10.1371/journal.pbio.3000242. eCollection 2019 Dec. PLoS Biol. 2019. PMID: 31805041 Free PMC article.
References
-
- Wilson E. O. Sociobiology: the new synthesis. Cambridge (Massachusetts); London: The Belknap Press of Harvard University Press; 2002.
-
- Wyatt T. D. Fifty years of pheromones. Nature. 2009;457:262–263. - PubMed
-
- de Bono M, Maricq A. V. Neuronal substrates of complex behaviors in C. elegans. Annu Rev Neurosci. 2005;28:451–501. - PubMed
-
- Butcher R. A, Fujita M, Schroeder F. C, Clardy J. Small-molecule pheromones that control dauer development in Caenorhabditis elegans. Nat Chem Biol. 2007;3:420–422. - PubMed
-
- Jeong P. Y, Jung M, Yim Y. H, Kim H, Park M, et al. Chemical structure and biological activity of the Caenorhabditis elegans dauer-inducing pheromone. Nature. 2005;433:541–545. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

