The relationship of thioredoxin-1 and cisplatin resistance: its impact on ROS and oxidative metabolism in lung cancer cells
- PMID: 22248473
- PMCID: PMC3326609
- DOI: 10.1158/1535-7163.MCT-11-0599
The relationship of thioredoxin-1 and cisplatin resistance: its impact on ROS and oxidative metabolism in lung cancer cells
Abstract
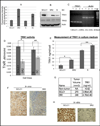
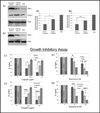
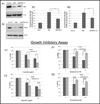
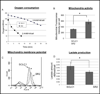
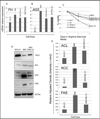
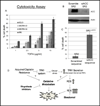
Elimination of cisplatin-resistant lung cancer cells remains a major obstacle. We have shown that cisplatin-resistant tumors have higher reactive oxygen species (ROS) levels and can be exploited for targeted therapy. Here, we show that increased secretion of the antioxidant thioredoxin-1 (TRX1) resulted in lowered intracellular TRX1 and contributed to higher ROS in cisplatin-resistant tumors in vivo and in vitro. By reconstituting TRX1 protein in cisplatin-resistant cells, we increased sensitivity to cisplatin but decreased sensitivity to elesclomol (ROS inducer). Conversely, decreased TRX1 protein in parental cells reduced the sensitivity to cisplatin but increased sensitivity to elesclomol. Cisplatin-resistant cells had increased endogenous oxygen consumption and mitochondrial activity but decreased lactic acid production. They also exhibited higher levels of argininosuccinate synthetase (ASS) and fumarase mRNA, which contributed to oxidative metabolism (OXMET) when compared with parental cells. Restoring intracellular TRX1 protein in cisplatin-resistant cells resulted in lowering ASS and fumarase mRNAs, which in turn sensitized them to arginine deprivation. Interestingly, cisplatin-resistant cells also had significantly higher basal levels of acetyl-CoA carboxylase (ACC) and fatty acid synthase (FAS). Overexpressing TRX1 lowered ACC and FAS proteins expressions in cisplatin-resistant cells. Chemical inhibition and short interfering RNA of ACC resulted in significant cell death in cisplatin-resistant compared with parental cells. Conversely, TRX1 overexpressed cisplatin-resistant cells resisted 5-(tetradecyloxy)-2-furoic acid (TOFA)-induced death. Collectively, lowering TRX1 expression through increased secretion leads cisplatin-resistant cells to higher ROS production and increased dependency on OXMET. These changes raise an intriguing therapeutic potential for future therapy in cisplatin-resistant lung cancer.
Figures
Similar articles
-
Cisplatin resistance in human cervical, ovarian and lung cancer cells.Cancer Chemother Pharmacol. 2015 Jun;75(6):1217-27. doi: 10.1007/s00280-015-2739-2. Epub 2015 Apr 18. Cancer Chemother Pharmacol. 2015. PMID: 25894720
-
Down-Regulation of Thioredoxin1 Is Involved in Death of Calu-6 Lung Cancer Cells Treated With Suberoyl Bishydroxamic Acid.J Cell Biochem. 2016 May;117(5):1250-61. doi: 10.1002/jcb.25409. Epub 2015 Nov 26. J Cell Biochem. 2016. PMID: 26460805
-
Suberoylanilide hydroxamic acid induces thioredoxin1-mediated apoptosis in lung cancer cells via up-regulation of miR-129-5p.Mol Carcinog. 2017 Dec;56(12):2566-2577. doi: 10.1002/mc.22701. Epub 2017 Aug 3. Mol Carcinog. 2017. PMID: 28667779
-
Exploiting ROS and metabolic differences to kill cisplatin resistant lung cancer.Oncotarget. 2017 Jul 25;8(30):49275-49292. doi: 10.18632/oncotarget.17568. Oncotarget. 2017. PMID: 28525376 Free PMC article.
-
Activation of mitochondrial oxidation by PDK2 inhibition reverses cisplatin resistance in head and neck cancer.Cancer Lett. 2016 Feb 1;371(1):20-9. doi: 10.1016/j.canlet.2015.11.023. Epub 2015 Nov 23. Cancer Lett. 2016. PMID: 26607904
Cited by
-
Mitochondrial and postmitochondrial survival signaling in cancer.Mitochondrion. 2014 May;16:18-25. doi: 10.1016/j.mito.2013.11.005. Epub 2013 Dec 10. Mitochondrion. 2014. PMID: 24333692 Free PMC article. Review.
-
The effect of FASN inhibition on the growth and metabolism of a cisplatin-resistant ovarian carcinoma model.Int J Cancer. 2018 Aug 15;143(4):992-1002. doi: 10.1002/ijc.31392. Epub 2018 Apr 1. Int J Cancer. 2018. PMID: 29569717 Free PMC article.
-
The role of peroxiredoxin II in chemoresistance of breast cancer cells.Breast Cancer (Dove Med Press). 2014 May 23;6:73-80. doi: 10.2147/BCTT.S61281. eCollection 2014. Breast Cancer (Dove Med Press). 2014. PMID: 24976757 Free PMC article. Review.
-
Genipin enhances the antitumor effect of elesclomol in A549 lung cancer cells by blocking uncoupling protein-2 and stimulating reactive oxygen species production.Oncol Lett. 2020 Dec;20(6):374. doi: 10.3892/ol.2020.12237. Epub 2020 Oct 21. Oncol Lett. 2020. PMID: 33154772 Free PMC article.
-
Protective Effects of Traditional Herbal Formulas on Cisplatin-Induced Nephrotoxicity in Renal Epithelial Cells via Antioxidant and Antiapoptotic Properties.Evid Based Complement Alternat Med. 2020 Aug 17;2020:5807484. doi: 10.1155/2020/5807484. eCollection 2020. Evid Based Complement Alternat Med. 2020. PMID: 32879634 Free PMC article.
References
-
- Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. - PubMed
-
- Witte AB, Anestal K, Jerremalm E, Ehrsson H, Arner ES. Inhibition of thioredoxin reductase but not of glutathione reductase by the major classes of alkylating and platinum-containing anticancer compounds. Free Radic Biol Med. 2005;39:696–703. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

