Natural and synthetic modulators of SK (K(ca)2) potassium channels inhibit magnesium-dependent activity of the kinase-coupled cation channel TRPM7
- PMID: 22242975
- PMCID: PMC3417452
- DOI: 10.1111/j.1476-5381.2012.01855.x
Natural and synthetic modulators of SK (K(ca)2) potassium channels inhibit magnesium-dependent activity of the kinase-coupled cation channel TRPM7
Abstract
Background and purpose: Transient receptor potential cation channel subfamily M member 7 (TRPM7) is a bifunctional protein comprising a TRP ion channel segment linked to an α-type protein kinase domain. TRPM7 is essential for proliferation and cell growth. Up-regulation of TRPM7 function is involved in anoxic neuronal death, cardiac fibrosis and tumour cell proliferation. The goal of this work was to identify non-toxic inhibitors of the TRPM7 channel and to assess the effect of blocking endogenous TRPM7 currents on the phenotype of living cells.
Experimental approach: We developed an aequorin bioluminescence-based assay of TRPM7 channel activity and performed a hypothesis-driven screen for inhibitors of the channel. The candidates identified were further assessed electrophysiologically and in cell biological experiments.
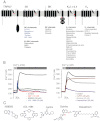
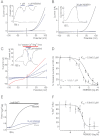
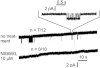
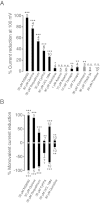
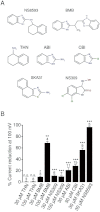
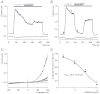
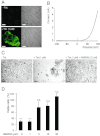
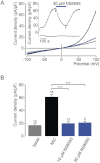
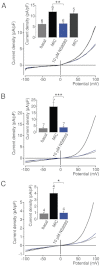
Key results: TRPM7 currents were inhibited by modulators of small conductance Ca²⁺ -activated K⁺ channels (K(Ca)2.1-2.3; SK) channels, including the antimalarial plant alkaloid quinine, CyPPA, dequalinium, NS8593, SKA31 and UCL 1684. The most potent compound NS8593 (IC₅₀ 1.6 µM) specifically targeted TRPM7 as compared with other TRP channels, interfered with Mg²⁺ -dependent regulation of TRPM7 channel and inhibited the motility of cultured cells. NS8593 exhibited full and reversible block of native TRPM7-like currents in HEK 293 cells, freshly isolated smooth muscle cells, primary podocytes and ventricular myocytes.
Conclusions and implications: This study reveals a tight overlap in the pharmacological profiles of TRPM7 and K(Ca)2.1-2.3 channels. NS8593 acts as a negative gating modulator of TRPM7 and is well-suited to study functional features and cellular roles of endogenous TRPM7.
© 2012 The Authors. British Journal of Pharmacology © 2012 The British Pharmacological Society.
Figures
Similar articles
-
Sphingosine and FTY720 are potent inhibitors of the transient receptor potential melastatin 7 (TRPM7) channels.Br J Pharmacol. 2013 Mar;168(6):1294-312. doi: 10.1111/bph.12012. Br J Pharmacol. 2013. PMID: 23145923 Free PMC article.
-
Mibefradil represents a new class of benzimidazole TRPM7 channel agonists.Pflugers Arch. 2016 Apr;468(4):623-34. doi: 10.1007/s00424-015-1772-7. Epub 2015 Dec 16. Pflugers Arch. 2016. PMID: 26669310
-
Inhibitory gating modulation of small conductance Ca2+-activated K+ channels by the synthetic compound (R)-N-(benzimidazol-2-yl)-1,2,3,4-tetrahydro-1-naphtylamine (NS8593) reduces afterhyperpolarizing current in hippocampal CA1 neurons.Mol Pharmacol. 2006 Nov;70(5):1771-82. doi: 10.1124/mol.106.027110. Epub 2006 Aug 22. Mol Pharmacol. 2006. PMID: 16926279
-
Mapping TRPM7 Function by NS8593.Int J Mol Sci. 2020 Sep 23;21(19):7017. doi: 10.3390/ijms21197017. Int J Mol Sci. 2020. PMID: 32977698 Free PMC article. Review.
-
Assessment of TRPM7 functions by drug-like small molecules.Cell Calcium. 2017 Nov;67:166-173. doi: 10.1016/j.ceca.2017.03.004. Epub 2017 Mar 14. Cell Calcium. 2017. PMID: 28356194 Review.
Cited by
-
Sphingosine and FTY720 are potent inhibitors of the transient receptor potential melastatin 7 (TRPM7) channels.Br J Pharmacol. 2013 Mar;168(6):1294-312. doi: 10.1111/bph.12012. Br J Pharmacol. 2013. PMID: 23145923 Free PMC article.
-
The Channel-Kinase TRPM7 as Novel Regulator of Immune System Homeostasis.Cells. 2018 Aug 17;7(8):109. doi: 10.3390/cells7080109. Cells. 2018. PMID: 30126133 Free PMC article. Review.
-
TRPM7 restrains plasmin activity and promotes transforming growth factor-β1 signaling in primary human lung fibroblasts.Arch Toxicol. 2022 Oct;96(10):2767-2783. doi: 10.1007/s00204-022-03342-x. Epub 2022 Jul 21. Arch Toxicol. 2022. PMID: 35864199 Free PMC article.
-
Mibefradil represents a new class of benzimidazole TRPM7 channel agonists.Pflugers Arch. 2016 Apr;468(4):623-34. doi: 10.1007/s00424-015-1772-7. Epub 2015 Dec 16. Pflugers Arch. 2016. PMID: 26669310
-
Physiological pathway of magnesium influx in rat ventricular myocytes.Biophys J. 2014 Nov 4;107(9):2049-58. doi: 10.1016/j.bpj.2014.09.015. Biophys J. 2014. PMID: 25418090 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

