IL-21 promotes the pathologic immune response to pneumovirus infection
- PMID: 22238461
- PMCID: PMC3277853
- DOI: 10.4049/jimmunol.1100767
IL-21 promotes the pathologic immune response to pneumovirus infection
Abstract
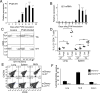
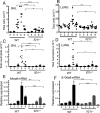
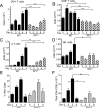
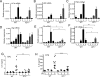
IL-21 is a cytokine with pleiotropic actions, promoting terminal differentiation of B cells, increased Ig production, and the development of Th17 and T follicular helper cells. IL-21 is also implicated in the development of autoimmune disease and has antitumor activity. In this study, we investigated the role of IL-21 in host defense to pneumonia virus of mice (PVM), which initiates an infection in mice resembling that of respiratory syncytial virus disease in humans. We found that PVM-infected mice expressed IL-21 in lung CD4(+) T cells. Following infection, Il21r(-/-) mice exhibited less lung infiltration by neutrophils than did wild-type (WT) mice and correspondingly had lower levels of the chemokine CXCL1 in bronchoalveolar lavage fluid and lung parenchyma. CD8(+), CD4(+), and γδ T cell numbers were also lower in the lungs of PVM-infected Il21r(-/-) mice than in infected WT mice, with normal Th17 cytokines but diminished IL-6 production in PVM-infected Il21r(-/-) mice. Strikingly, Il21r(-/-) mice had enhanced survival following PVM infection, and moreover, treatment of WT mice with soluble IL-21R-Fc fusion protein enhanced their survival. These data reveal that IL-21 promotes the pathogenic inflammatory effect of PVM and indicate that manipulating IL-21 signaling may represent an immunomodulatory strategy for controlling PVM and potentially other respiratory virus infections.
Figures




Similar articles
-
Pulmonary eosinophils and their role in immunopathologic responses to formalin-inactivated pneumonia virus of mice.J Immunol. 2009 Jul 1;183(1):604-12. doi: 10.4049/jimmunol.0802270. J Immunol. 2009. PMID: 19542471 Free PMC article.
-
Critical Adverse Impact of IL-6 in Acute Pneumovirus Infection.J Immunol. 2019 Feb 1;202(3):871-882. doi: 10.4049/jimmunol.1800927. Epub 2018 Dec 21. J Immunol. 2019. PMID: 30578308 Free PMC article.
-
Protective role of P2Y2 receptor against lung infection induced by pneumonia virus of mice.PLoS One. 2012;7(11):e50385. doi: 10.1371/journal.pone.0050385. Epub 2012 Nov 21. PLoS One. 2012. PMID: 23185614 Free PMC article.
-
The Pneumonia Virus of Mice (PVM) model of acute respiratory infection.Viruses. 2012 Dec;4(12):3494-510. doi: 10.3390/v4123494. Viruses. 2012. PMID: 23342367 Free PMC article. Review.
-
Pneumonia virus of mice: severe respiratory infection in a natural host.Immunol Lett. 2008 Jun 15;118(1):6-12. doi: 10.1016/j.imlet.2008.03.013. Epub 2008 Apr 22. Immunol Lett. 2008. PMID: 18471897 Free PMC article. Review.
Cited by
-
IL-21 promotes myocardial ischaemia/reperfusion injury through the modulation of neutrophil infiltration.Br J Pharmacol. 2018 Apr;175(8):1329-1343. doi: 10.1111/bph.13781. Epub 2017 Apr 12. Br J Pharmacol. 2018. PMID: 28294304 Free PMC article.
-
Essentials of Th17 cell commitment and plasticity.Blood. 2013 Mar 28;121(13):2402-14. doi: 10.1182/blood-2012-09-378653. Epub 2013 Jan 16. Blood. 2013. PMID: 23325835 Free PMC article. Review.
-
IL-21 optimizes T cell and humoral responses in the central nervous system during viral encephalitis.J Neuroimmunol. 2013 Oct 15;263(1-2):43-54. doi: 10.1016/j.jneuroim.2013.07.019. Epub 2013 Aug 6. J Neuroimmunol. 2013. PMID: 23992866 Free PMC article.
-
Microdevices for examining immunological responses of single cells to HIV.Biosci Rep. 2014 Aug 18;34(4):e00134. doi: 10.1042/BSR20140097. Biosci Rep. 2014. PMID: 25028990 Free PMC article. Review.
-
IL-21 Promotes Pulmonary Fibrosis through the Induction of Profibrotic CD8+ T Cells.J Immunol. 2015 Dec 1;195(11):5251-60. doi: 10.4049/jimmunol.1500777. Epub 2015 Oct 30. J Immunol. 2015. PMID: 26519529 Free PMC article.
References
-
- Spolski R, Leonard WJ. Interleukin-21: Basic Biology and Implications for Cancer and Autoimmunity. Annu Rev Immunol. 2008;26:57–79. - PubMed
-
- Leonard WJ. Cytokines and immunodeficiency diseases. Nat Rev Immunol. 2001;1:200–208. - PubMed
-
- Noguchi M, Yi H, Rosenblatt HM, Filipovich AH, Adelstein S, Modi WS, McBride OW, Leonard WJ. Interleukin-2 receptor gamma chain mutation results in X-linked severe combined immunodeficiency in humans. Cell. 1993;73:147–157. - PubMed
-
- Ozaki K, Spolski R, Ettinger R, Kim HP, Wang G, Qi CF, Hwu P, Shaffer DJ, Akilesh S, Roopenian DC, Morse HC, 3rd, Lipsky PE, Leonard WJ. Regulation of B cell differentiation and plasma cell generation by IL-21, a novel inducer of Blimp-1 and Bcl-6. J Immunol. 2004;173:5361–5371. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

