Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy
- PMID: 22238332
- PMCID: PMC3720836
- DOI: 10.1126/scitranslmed.3002693
Detection of 2-hydroxyglutarate in IDH-mutated glioma patients by in vivo spectral-editing and 2D correlation magnetic resonance spectroscopy
Abstract
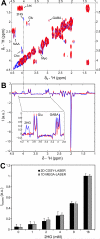
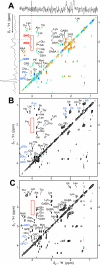
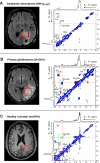
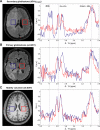
Mutations in the gene isocitrate dehydrogenase 1 (IDH1) are present in up to 86% of grade II and III gliomas and secondary glioblastoma. Arginine 132 (R132) mutations in the enzyme IDH1 result in excess production of the metabolite 2-hydroxyglutarate (2HG), which could be used as a biomarker for this subset of gliomas. Here, we use optimized in vivo spectral-editing and two-dimensional (2D) correlation magnetic resonance spectroscopy (MRS) methods to unambiguously detect 2HG noninvasively in glioma patients with IDH1 mutations. By comparison, fitting of conventional 1D MR spectra can provide false-positive readouts owing to spectral overlap of 2HG and chemically similar brain metabolites, such as glutamate and glutamine. 2HG was also detected using 2D high-resolution magic angle spinning MRS performed ex vivo on a separate set of glioma biopsy samples. 2HG detection by in vivo or ex vivo MRS enabled detailed molecular characterization of a clinically important subset of human gliomas. This has implications for diagnosis as well as monitoring of treatments targeting mutated IDH1.
Figures
Similar articles
-
Diagnostic value of glutamate with 2-hydroxyglutarate in magnetic resonance spectroscopy for IDH1 mutant glioma.Neuro Oncol. 2016 Nov;18(11):1559-1568. doi: 10.1093/neuonc/now090. Epub 2016 May 5. Neuro Oncol. 2016. PMID: 27154922 Free PMC article.
-
Magnetic resonance of 2-hydroxyglutarate in IDH1-mutated low-grade gliomas.Sci Transl Med. 2012 Jan 11;4(116):116ra5. doi: 10.1126/scitranslmed.3002796. Sci Transl Med. 2012. PMID: 22238333 Free PMC article.
-
Noninvasive assessment of isocitrate dehydrogenase mutation status in cerebral gliomas by magnetic resonance spectroscopy in a clinical setting.J Neurosurg. 2018 Feb;128(2):391-398. doi: 10.3171/2016.10.JNS161793. Epub 2017 Mar 3. J Neurosurg. 2018. PMID: 28298040
-
Detection of oncogenic IDH1 mutations using magnetic resonance spectroscopy of 2-hydroxyglutarate.J Clin Invest. 2013 Sep;123(9):3659-63. doi: 10.1172/JCI67229. Epub 2013 Sep 3. J Clin Invest. 2013. PMID: 23999439 Free PMC article. Review.
-
In-Vivo Proton Magnetic Resonance Spectroscopy of 2-Hydroxyglutarate in Isocitrate Dehydrogenase-Mutated Gliomas: A Technical Review for Neuroradiologists.Korean J Radiol. 2016 Sep-Oct;17(5):620-32. doi: 10.3348/kjr.2016.17.5.620. Epub 2016 Aug 23. Korean J Radiol. 2016. PMID: 27587950 Free PMC article. Review.
Cited by
-
Improved localisation for 2-hydroxyglutarate detection at 3T using long-TE semi-LASER.Tomography. 2016 Jun;2(2):94-105. doi: 10.18383/j.tom.2016.00139. Tomography. 2016. PMID: 27547821 Free PMC article.
-
Metabolomic profiling of hormone-dependent cancers: a bird's eye view.Trends Endocrinol Metab. 2015 Sep;26(9):477-85. doi: 10.1016/j.tem.2015.07.001. Epub 2015 Aug 1. Trends Endocrinol Metab. 2015. PMID: 26242817 Free PMC article. Review.
-
Volumetric relationship between 2-hydroxyglutarate and FLAIR hyperintensity has potential implications for radiotherapy planning of mutant IDH glioma patients.Neuro Oncol. 2016 Nov;18(11):1569-1578. doi: 10.1093/neuonc/now100. Epub 2016 Jul 5. Neuro Oncol. 2016. PMID: 27382115 Free PMC article.
-
Magnetic resonance spectroscopy of isocitrate dehydrogenase mutated gliomas: current knowledge on the neurochemical profile.Curr Opin Neurol. 2020 Aug;33(4):413-421. doi: 10.1097/WCO.0000000000000833. Curr Opin Neurol. 2020. PMID: 32657882 Free PMC article. Review.
-
Isocitrate dehydrogenase 1: what it means to the neurosurgeon: a review.J Neurosurg. 2013 Jun;118(6):1176-80. doi: 10.3171/2013.3.JNS122282. Epub 2013 Apr 12. J Neurosurg. 2013. PMID: 23581583 Free PMC article. Review.
References
-
- Parsons DW, Jones S, Zhang XS, Lin JCH, Leary RJ, Angenendt P, Mankoo P, Carter H, Siu IM, Gallia GL, Olivi A, McLendon R, Rasheed BA, Keir S, Nikolskaya T, Nikolsky Y, Busam DA, Tekleab H, Diaz LA, Hartigan J, Smith DR, Strausberg RL, Marie SKN, Shinjo SMO, Yan H, Riggins GJ, Bigner DD, Karchin R, Papadopoulos N, Parmigiani G, Vogelstein B, Velculescu VE, Kinzler KW. Science. 2008 Sep;321:1807. - PMC - PubMed
-
- Chin L, Meyerson M, Aldape K, Bigner D, Mikkelsen T, VandenBerg S, Kahn A, Penny R, Ferguson ML, Gerhard DS, Getz G, Brennan C, Taylor BS, Winckler W, Park P, Ladanyi M, Hoadley KA, Verhaak RGW, Hayes DN, Spellman PT, Absher D, Weir BA, Ding L, Wheeler D, Lawrence MS, Cibulskis K, Mardis E, Zhang JH, Wilson RK, Donehower L, Wheeler DA, Purdom E, Wallis J, Laird PW, Herman JG, Schuebel KE, Weisenberger DJ, Baylin SB, Schultz N, Yao J, Wiedemeyer R, Weinstein J, Sander C, Gibbs RA, Gray J, Kucherlapati R, Lander ES, Myers RM, Perou CM, McLendon R, Friedman A, Van Meir EG, Brat DJ, Mastrogianakis GM, Olson JJ, Lehman N, Yung WKA, Bogler O, Berger M, Prados M, Muzny D, Morgan M, Scherer S, Sabo A, Nazareth L, Lewis L, Hall O, Zhu YM, Ren YR, Alvi O, Yao JQ, Hawes A, Jhangiani S, Fowler G, San Lucas A, Kovar C, Cree A, Dinh H, Santibanez J, Joshi V, Gonzalez-Garay ML, Miller CA, Milosavljevic A, Sougnez C, Fennell T, Mahan S, Wilkinson J, Ziaugra L, Onofrio R, Bloom T, Nicol R, Ardlie K, Baldwin J, Gabriel S, Fulton RS, McLellan MD, Larson DE, Shi XQ, Abbott R, Fulton L, Chen K, Koboldt DC, Wendl MC, Meyer R, Tang YZ, Lin L, Osborne JR, Dunford-Shore BH, Miner TL, Delehaunty K, Markovic C, Swift G, Courtney W, Pohl C, Abbott S, Hawkins A, Leong S, Haipek C, Schmidt H, Wiechert M, Vickery T, Scott S, Dooling DJ, Chinwalla A, Weinstock GM, O'Kelly M, Robinson J, Alexe G, Beroukhim R, Carter S, Chiang D, Gould J, Gupta S, Korn J, Mermel C, Mesirov J, Monti S, Nguyen H, Parkin M, Reich M, Stransky N, Garraway L, Golub T, Protopopov A, Perna I, Aronson S, Sathiamoorthy N, Ren G, Kim H, Kong SK, Xiao YH, Kohane IS, Seidman J, Cope L, Pan F, Van Den Berg D, Van Neste L, Yi JM, Li JZ, Southwick A, Brady S, Aggarwal A, Chung T, Sherlock G, Brooks JD, Jakkula LR, Lapuk AV, Marr H, Dorton S, Choi YG, Han J, Ray A, Wang V, Durinck S, Robinson M, Wang NJ, Vranizan K, Peng V, Van Name E, Fontenay GV, Ngai J, Conboy JG, Parvin B, Feiler HS, Speed TP, Socci ND, Olshen A, Lash A, Reva B, Antipin Y, Stukalov A, Gross B, Cerami E, Wang WQ, Qin LX, Seshan VE, Villafania L, Cavatore M, Borsu L, Viale A, Gerald W, Topal MD, Qi Y, Balu S, Shi Y, Wu G, Bittner M, Shelton T, Lenkiewicz E, Morris S, Beasley D, Sanders S, Sfeir R, Chen J, Nassau D, Feng L, Hickey E, Schaefer C, Madhavan S, Buetow K, Barker A, Vockley J, Compton C, Vaught J, Fielding P, Collins F, Good P, Guyer M, Ozenberger B, Peterson J, Thomson E. Nature. 2008 Oct;455:1061. - PubMed
-
- Kloosterhof NK, Bralten LBC, Dubbink HJ, French PJ, van den Bent MJ. Lancet Oncology. 2011 Jan;12:83. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

