Engineering an integrated cellular interface in three-dimensional hydrogel cultures permits monitoring of reciprocal astrocyte and neuronal responses
- PMID: 22235832
- PMCID: PMC3381295
- DOI: 10.1089/ten.TEC.2011.0587
Engineering an integrated cellular interface in three-dimensional hydrogel cultures permits monitoring of reciprocal astrocyte and neuronal responses
Abstract
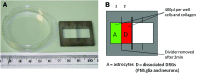
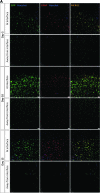
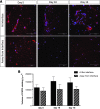
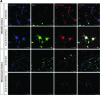
This study reports a new type of three-dimensional (3D) tissue model for studying interactions between cell types in collagen hydrogels. The aim was to create a 3D cell culture model containing separate cell populations in close proximity without the presence of a mechanical barrier, and demonstrate its relevance to modeling the axon growth-inhibitory cellular interfaces that develop in the central nervous system (CNS) in response to damage. This provides a powerful new tool to determine which aspects of the astroglial scar response and subsequent neuronal regeneration inhibition are determined by the presence of the other cell types. Astrocytes (CNS glia) and dissociated dorsal root ganglia (DRG; containing neurons and peripheral nervous system [PNS] glia) were seeded within collagen solution at 4 °C in adjacent chambers of a stainless steel mould, using cells cultured from wild-type or green fluorescent protein expressing rats, to track specific populations. The divider between the chambers was removed using a protocol that allowed the gels to integrate without mixing of the cell populations. Following setting of the gels, they were maintained in culture for up to 15 days. Reciprocal astrocyte and neuronal responses were monitored using confocal microscopy and 3D image analysis. At DRG:astrocyte interfaces, by 5 days there was an increase in the number of astrocytes at the interface followed by hypertrophy and increased glial fibrillary acidic protein expression at 10 and 15 days, indicative of reactive gliosis. Neurons avoided crossing DRG:astrocyte interfaces, and neuronal growth was restricted to the DRG part of the gel. By contrast, neurons were able to grow freely across DRG:DRG interfaces, demonstrating the absence of a mechanical barrier. These results show that in a precisely controlled 3D environment, an interface between DRG and astrocyte cultures is sufficient to trigger reactive gliosis and inhibition of neuronal regeneration across the interface. Different aspects of the astrocyte response could be independently monitored, providing an insight into the formation of a glial scar. This technology has wide potential for researchers wishing to maintain and monitor interactions between adjacent cell populations in 3D culture.
Figures




Similar articles
-
A cortical astrocyte subpopulation inhibits axon growth in vitro and in vivo.Mol Med Rep. 2015 Aug;12(2):2598-606. doi: 10.3892/mmr.2015.3702. Epub 2015 Apr 29. Mol Med Rep. 2015. PMID: 25936767 Free PMC article.
-
A new in vitro model of the glial scar inhibits axon growth.Glia. 2008 Nov 15;56(15):1691-709. doi: 10.1002/glia.20721. Glia. 2008. PMID: 18618667 Free PMC article.
-
A versatile 3D culture model facilitates monitoring of astrocytes undergoing reactive gliosis.J Tissue Eng Regen Med. 2009 Dec;3(8):634-46. doi: 10.1002/term.209. J Tissue Eng Regen Med. 2009. PMID: 19813215 Free PMC article.
-
Reciprocal modulation between microglia and astrocyte in reactive gliosis following the CNS injury.Mol Neurobiol. 2013 Dec;48(3):690-701. doi: 10.1007/s12035-013-8460-4. Epub 2013 Apr 24. Mol Neurobiol. 2013. PMID: 23613214 Free PMC article. Review.
-
Biomaterial Approaches to Modulate Reactive Astroglial Response.Cells Tissues Organs. 2018;205(5-6):372-395. doi: 10.1159/000494667. Epub 2018 Dec 5. Cells Tissues Organs. 2018. PMID: 30517922 Free PMC article. Review.
Cited by
-
Glucose-coated gold nanoparticles transfer across human brain endothelium and enter astrocytes in vitro.PLoS One. 2013 Dec 5;8(12):e81043. doi: 10.1371/journal.pone.0081043. eCollection 2013. PLoS One. 2013. PMID: 24339894 Free PMC article.
-
Advances in ex vivo models and lab-on-a-chip devices for neural tissue engineering.Biomaterials. 2019 Apr;198:146-166. doi: 10.1016/j.biomaterials.2018.05.012. Epub 2018 May 11. Biomaterials. 2019. PMID: 29880219 Free PMC article. Review.
-
The role of neural stem cells in regulating glial scar formation and repair.Cell Tissue Res. 2022 Mar;387(3):399-414. doi: 10.1007/s00441-021-03554-0. Epub 2021 Nov 25. Cell Tissue Res. 2022. PMID: 34820704 Free PMC article. Review.
-
Three Dimensional Conjugation of Recombinant N-Cadherin to a Hydrogel for In Vitro Anisotropic Neural Growth.J Mater Chem B. 2016 Nov 14;4(42):6803-6811. doi: 10.1039/C6TB01814A. Epub 2016 Sep 21. J Mater Chem B. 2016. PMID: 28503305 Free PMC article.
-
Bioprinting Neural Systems to Model Central Nervous System Diseases.Adv Funct Mater. 2020 Oct 28;30(44):1910250. doi: 10.1002/adfm.201910250. Epub 2020 Apr 22. Adv Funct Mater. 2020. PMID: 34566552 Free PMC article. Review.
References
-
- East E. Phillips J.B. Tissue engineered cell culture models for nervous system research. In: Greco G.N., editor. Tissue Engineering Research Trends. New York: Nova Science Publishers; 2008. pp. 141–160.
-
- Kaewkhaw R. Scutt A.M. Haycock J.W. Anatomical site influences the differentiation of adipose-derived stem cells for Schwann-cell phenotype and function. Glia. 2011;59:734. - PubMed
-
- LaPlaca M.C. Simon C.M. Prado G.R. Cullen D.K. CNS injury biomechanics and experimental models. Prog Brain Res. 2007;161:13. - PubMed
-
- King V.R. Phillips J.B. Hunt-Grubbe H. Brown R. Priestley J.V. Characterization of non-neuronal elements within fibronectin mats implanted into the damaged adult rat spinal cord. Biomaterials. 2006;27:485. - PubMed
-
- Phillips J.B. King V.R. Ward Z. Porter R.A. Priestley J.V. Brown R.A. Fluid shear in viscous fibronectin gels allows aggregation of fibrous materials for CNS tissue engineering. Biomaterials. 2004;25:2769. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

