A photochemical approach for controlled drug release in targeted drug delivery
- PMID: 22225916
- PMCID: PMC3267001
- DOI: 10.1016/j.bmc.2011.12.020
A photochemical approach for controlled drug release in targeted drug delivery
Abstract
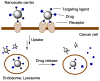
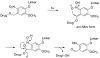
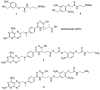
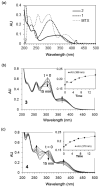
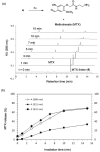
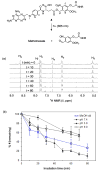
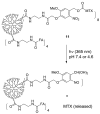
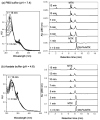
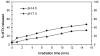
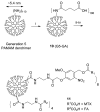
Photochemistry provides a unique mechanism that enables the active control of drug release in cancer-targeting drug delivery. This study investigates the light-mediated release of methotrexate, an anticancer drug, using a photocleavable linker strategy based on o-nitrobenzyl protection. We evaluated two types of the o-nitrobenzyl-linked methotrexate for the drug release study and further extended the study to a fifth-generation poly(amidoamine) dendrimer carrier covalently conjugated with methotrexate via the o-nitrobenzyl linker. We performed the drug release studies by using a combination of three standard analytical methods that include UV/vis spectrometry, (1)H NMR spectroscopy, and anal. HPLC. This article reports that methotrexate is released by the photochemical mechanism in an actively controlled manner. The rate of the drug release varies in response to multiple control parameters, including linker design, light wavelength, exposure time, and the pH of the medium where the drug release occurs.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures

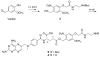
Similar articles
-
Targeted drug delivery with dendrimers: comparison of the release kinetics of covalently conjugated drug and non-covalent drug inclusion complex.Adv Drug Deliv Rev. 2005 Dec 14;57(15):2203-14. doi: 10.1016/j.addr.2005.09.014. Epub 2005 Nov 14. Adv Drug Deliv Rev. 2005. PMID: 16290254
-
Targeted delivery of methotrexate to epidermal growth factor receptor-positive brain tumors by means of cetuximab (IMC-C225) dendrimer bioconjugates.Mol Cancer Ther. 2006 Jan;5(1):52-9. doi: 10.1158/1535-7163.MCT-05-0325. Mol Cancer Ther. 2006. PMID: 16432162
-
Double targeting and aptamer-assisted controlled release delivery of epirubicin to cancer cells by aptamers-based dendrimer in vitro and in vivo.Eur J Pharm Biopharm. 2016 May;102:152-8. doi: 10.1016/j.ejpb.2016.03.013. Epub 2016 Mar 14. Eur J Pharm Biopharm. 2016. PMID: 26987703
-
Mechanisms and implications of dual-acting methotrexate in folate-targeted nanotherapeutic delivery.Int J Mol Sci. 2015 Jan 13;16(1):1772-90. doi: 10.3390/ijms16011772. Int J Mol Sci. 2015. PMID: 25590303 Free PMC article. Review.
-
Poly (amidoamine) (PAMAM) dendrimer mediated delivery of drug and pDNA/siRNA for cancer therapy.Int J Pharm. 2018 Jul 30;546(1-2):215-225. doi: 10.1016/j.ijpharm.2018.05.045. Epub 2018 May 19. Int J Pharm. 2018. PMID: 29787895 Review.
Cited by
-
Porous Silicon Nanocarriers with Stimulus-Cleavable Linkers for Effective Cancer Therapy.Adv Healthc Mater. 2022 Jun;11(12):e2200076. doi: 10.1002/adhm.202200076. Epub 2022 Apr 3. Adv Healthc Mater. 2022. PMID: 35306736 Free PMC article.
-
PAMAM dendrimers as efficient drug and gene delivery nanosystems for cancer therapy.Appl Mater Today. 2018 Sep;12:177-190. doi: 10.1016/j.apmt.2018.05.002. Epub 2018 May 29. Appl Mater Today. 2018. PMID: 30511014 Free PMC article.
-
Design and in vitro validation of multivalent dendrimer methotrexates as a folate-targeting anticancer therapeutic.Curr Pharm Des. 2013;19(37):6594-605. doi: 10.2174/1381612811319370004. Curr Pharm Des. 2013. PMID: 23621534 Free PMC article.
-
Folic Acid Antimetabolites (Antifolates): A Brief Review on Synthetic Strategies and Application Opportunities.Molecules. 2022 Sep 22;27(19):6229. doi: 10.3390/molecules27196229. Molecules. 2022. PMID: 36234766 Free PMC article. Review.
-
A Photo-degradable Crosslinker for the Development of Light-responsive Protocell Membranes.Chemistry. 2023 Nov 2;29(61):e202302058. doi: 10.1002/chem.202302058. Epub 2023 Sep 22. Chemistry. 2023. PMID: 37497813 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

