Human steroid biosynthesis for the oncologist
- PMID: 22222232
- PMCID: PMC3653186
- DOI: 10.2310/JIM.0b013e3182408567
Human steroid biosynthesis for the oncologist
Abstract
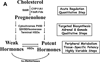
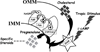
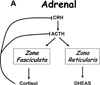
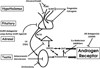
In 2005, results from the Arimidex, Tamoxifen Alone or in Combination (ATAC) trial ushered in a new era of endocrine therapy for hormone-responsive malignancies. This study demonstrated that, compared with tamoxifen (a selective estrogen receptor modulator), anastrozole (aromatase inhibitor [AI]) prolonged time to recurrence and disease-free survival for postmenopausal women with breast cancer. The advantage was even greater for those with estrogen receptor-positive (ER) tumors, and anastrozole was better tolerated than tamoxifen. Since then, AIs have become first-line adjuvant therapy for ER breast cancer in postmenopausal women.In late 2010, a trial comparing abiraterone acetate (a 17-hydroxylase/17,20-lyase [CYP17A1] inhibitor) plus prednisone versus prednisone alone in men with castration-resistant prostate cancer (CRPC) previously treated with docetaxel chemotherapy was terminated early because of the survival benefit in the abiraterone acetate arm. This result not only validated a new therapy for CRPC but also, with the antecedent phase I-II abiraterone studies, shattered our understanding of the molecular mechanisms underpinning CRPC development and progression.Aromatase inhibitors and CYP17A1 inhibitors will be widely used by oncologists, yet fellowship programs provide little training in steroid biosynthesis, compared with training in the biology of standard chemotherapies. Consequently, these drugs might be used without an appreciation of their caveats and pitfalls. The purpose of this review was to acquaint practicing oncologists with the fundamental principles and pathways of steroid biosynthesis, to improve their understanding of how and why these drugs work, and to alert these physicians to potential problems related to the drugs' mechanisms of action.
Figures






Similar articles
-
Sequential hormonal therapy for metastatic breast cancer after adjuvant tamoxifen or anastrozole.Breast Cancer Res Treat. 2003;80 Suppl 1:S19-26; discussion S27-8. doi: 10.1023/a:1025459232293. Breast Cancer Res Treat. 2003. PMID: 14535531 Review.
-
The role of aromatase inhibitors in early breast cancer.Curr Treat Options Oncol. 2003 Apr;4(2):133-40. doi: 10.1007/s11864-003-0014-y. Curr Treat Options Oncol. 2003. PMID: 12594939 Review.
-
Challenges in the endocrine management of breast cancer.Breast. 2003 Aug;12 Suppl 2:S2-19. doi: 10.1016/s0960-9776(03)80158-3. Breast. 2003. PMID: 14659138 Review.
-
Emerging role of aromatase inhibitors in the adjuvant setting.Am J Clin Oncol. 2003 Aug;26(4):S27-33. doi: 10.1097/00000421-200308001-00005. Am J Clin Oncol. 2003. PMID: 12902874 Review.
-
Data from the Arimidex, tamoxifen, alone or in combination (ATAC) trial: implications for use of aromatase inhibitors in 2003.Clin Cancer Res. 2004 Jan 1;10(1 Pt 2):355S-61S. doi: 10.1158/1078-0432.ccr-031203. Clin Cancer Res. 2004. PMID: 14734491
Cited by
-
Catalytically relevant electrostatic interactions of cytochrome P450c17 (CYP17A1) and cytochrome b5.J Biol Chem. 2014 Dec 5;289(49):33838-49. doi: 10.1074/jbc.M114.608919. Epub 2014 Oct 14. J Biol Chem. 2014. PMID: 25315771 Free PMC article.
-
Critical Roles of SRC-3 in the Development and Progression of Breast Cancer, Rendering It a Prospective Clinical Target.Cancers (Basel). 2023 Oct 31;15(21):5242. doi: 10.3390/cancers15215242. Cancers (Basel). 2023. PMID: 37958417 Free PMC article. Review.
-
Proteomic analysis revealed common, unique and systemic signatures in gender-dependent hepatocarcinogenesis.Biol Sex Differ. 2020 Aug 13;11(1):46. doi: 10.1186/s13293-020-00316-5. Biol Sex Differ. 2020. PMID: 32792008 Free PMC article.
-
Estrogen/ER in anti-tumor immunity regulation to tumor cell and tumor microenvironment.Cancer Cell Int. 2021 Jun 7;21(1):295. doi: 10.1186/s12935-021-02003-w. Cancer Cell Int. 2021. PMID: 34098945 Free PMC article. Review.
-
Ru(ii) polypyridyl complexes as photocages for bioactive compounds containing nitriles and aromatic heterocycles.Chem Commun (Camb). 2018 Feb 1;54(11):1280-1290. doi: 10.1039/c7cc09000e. Chem Commun (Camb). 2018. PMID: 29323683 Free PMC article.
References
-
- Boyd S. On oophorectomy in cancer of the breast. BMJ. 1900;2:1161–1167.
-
- Huggins C. Prostatic cancer treated by orchiectomy; the five year results. JAMA. 1946;15:576–581. - PubMed
-
- Huggins C, Dao TL. Adrenalectomy and oophorectomy in treatment of advanced carcinoma of the breast. JAMA. 1953;18:1388–1394. - PubMed
-
- Santen RJ, Worgul TJ, Samojlik E, et al. A randomized trial comparing surgical adrenalectomy with aminoglutethimide plus hydrocortisone in women with advanced breast cancer. N Engl J Med. 1981;305:545–551. - PubMed
-
- Susan LP, Roth RB, Adkins WC. Regression of prostate cancer metastasis by high doses of diethylsilbestrol phosphate. Urology. 1976;7:598–601. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

