Double mutation in photosystem II reaction centers and elevated CO2 grant thermotolerance to mesophilic cyanobacterium
- PMID: 22216094
- PMCID: PMC3245225
- DOI: 10.1371/journal.pone.0028389
Double mutation in photosystem II reaction centers and elevated CO2 grant thermotolerance to mesophilic cyanobacterium
Abstract
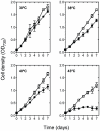
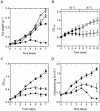
Photosynthetic biomass production rapidly declines in mesophilic cyanobacteria grown above their physiological temperatures largely due to the imbalance between degradation and repair of the D1 protein subunit of the heat susceptible Photosystem II reaction centers (PSIIRC). Here we show that simultaneous replacement of two conserved residues in the D1 protein of the mesophilic Synechocystis sp. PCC 6803, by the analogue residues present in the thermophilic Thermosynechococcus elongatus, enables photosynthetic growth, extensive biomass production and markedly enhanced stability and repair rate of PSIIRC for seven days even at 43 °C but only at elevated CO(2) (1%). Under the same conditions, the Synechocystis control strain initially presented very slow growth followed by a decline after 3 days. Change in the thylakoid membrane lipids, namely the saturation of the fatty acids is observed upon incubation for the different strains, but only the double mutant shows a concomitant major change of the enthalpy and entropy for the light activated Q(A)(-)→Q(B) electron transfer, rendering them similar to those of the thermophilic strain. Following these findings, computational chemistry and protein dynamics simulations we propose that the D1 double mutation increases the folding stability of the PSIIRC at elevated temperatures. This, together with the decreased impairment of D1 protein repair under increased CO(2) concentrations result in the observed photothermal tolerance of the photosynthetic machinery in the double mutant.
© 2011 Dinamarca et al.
Conflict of interest statement
Figures

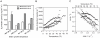

Similar articles
-
Characterization of mutants expressing thermostable D1 and D2 polypeptides of photosystem II in the cyanobacterium Synechococcus elongatus PCC 7942.J Biosci Bioeng. 2018 Oct;126(4):417-424. doi: 10.1016/j.jbiosc.2018.04.015. Epub 2018 Jun 8. J Biosci Bioeng. 2018. PMID: 29891421
-
Slr2013 is a novel protein regulating functional assembly of photosystem II in Synechocystis sp. strain PCC 6803.J Bacteriol. 2003 Nov;185(22):6615-23. doi: 10.1128/JB.185.22.6615-6623.2003. J Bacteriol. 2003. PMID: 14594835 Free PMC article.
-
FtsH is involved in the early stages of repair of photosystem II in Synechocystis sp PCC 6803.Plant Cell. 2003 Sep;15(9):2152-64. doi: 10.1105/tpc.012609. Plant Cell. 2003. PMID: 12953117 Free PMC article.
-
Some Photosystem II properties depending on the D1 protein variants in Thermosynechococcus elongatus.Biochim Biophys Acta. 2014 Sep;1837(9):1427-34. doi: 10.1016/j.bbabio.2013.12.011. Epub 2014 Jan 2. Biochim Biophys Acta. 2014. PMID: 24388918 Review.
-
[High-temperature adaptation mechanisms and biotechnological potentials of thermophilic cyanobacteria].Sheng Wu Gong Cheng Xue Bao. 2024 Jun 25;40(6):1752-1775. doi: 10.13345/j.cjb.230645. Sheng Wu Gong Cheng Xue Bao. 2024. PMID: 38914490 Review. Chinese.
Cited by
-
Bioprospecting the thermal waters of the Roman baths: isolation of oleaginous species and analysis of the FAME profile for biodiesel production.AMB Express. 2013 Jan 31;3(1):9. doi: 10.1186/2191-0855-3-9. AMB Express. 2013. PMID: 23369619 Free PMC article.
-
Targeting mutations to the plastidial psbA gene of Chlamydomonas reinhardtii without direct positive selection.Sci Rep. 2019 May 14;9(1):7367. doi: 10.1038/s41598-019-42617-9. Sci Rep. 2019. PMID: 31089169 Free PMC article.
-
Temperature dependence of photosynthetic reaction centre activity in Rhodospirillum rubrum.Photosynth Res. 2019 Nov;142(2):181-193. doi: 10.1007/s11120-019-00652-7. Epub 2019 Jul 2. Photosynth Res. 2019. PMID: 31267356 Free PMC article.
-
A single residue controls electron transfer gating in photosynthetic reaction centers.Sci Rep. 2017 Mar 16;7:44580. doi: 10.1038/srep44580. Sci Rep. 2017. PMID: 28300167 Free PMC article.
-
Enhanced thermal stability of the thylakoid membranes from spruce. A comparison with selected angiosperms.Photosynth Res. 2016 Dec;130(1-3):357-371. doi: 10.1007/s11120-016-0269-3. Epub 2016 May 6. Photosynth Res. 2016. PMID: 27154572
References
-
- Szilard A, Sass L, Hideg E, Vass I. Photoinactivation of photosystem II by flashing light. Photosynth Res. 2005;84:15–20. - PubMed
-
- Vass I, Cser K. Janus-faced charge recombinations in photosystem II photoinhibition. Trends Plant Sci. 2009;14:200–205. - PubMed
-
- Aro EM, Suorsa M, Rokka A, Allahverdiyeva Y, Paakkarinen V, et al. Dynamics of photosystem II: a proteomic approach to thylakoid protein complexes. J Exp Bot. 2005;56:347–356. - PubMed
-
- Edelman M, Mattoo AK. D1-protein dynamics in photosystem II: the lingering enigma. Photosynth Res. 2008;98:609–620. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

