In vitro blood-brain barrier models: current and perspective technologies
- PMID: 22213383
- PMCID: PMC3288147
- DOI: 10.1002/jps.23022
In vitro blood-brain barrier models: current and perspective technologies
Abstract
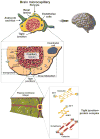
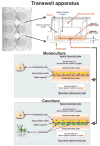
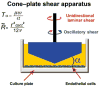
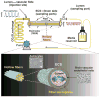
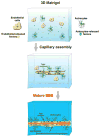
Even in the 21st century, studies aimed at characterizing the pathological paradigms associated with the development and progression of central nervous system diseases are primarily performed in laboratory animals. However, limited translational significance, high cost, and labor to develop the appropriate model (e.g., transgenic or inbred strains) have favored parallel in vitro approaches. In vitro models are of particular interest for cerebrovascular studies of the blood-brain barrier (BBB), which plays a critical role in maintaining the brain homeostasis and neuronal functions. Because the BBB dynamically responds to many events associated with rheological and systemic impairments (e.g., hypoperfusion), including the exposure of potentially harmful xenobiotics, the development of more sophisticated artificial systems capable of replicating the vascular properties of the brain microcapillaries are becoming a major focus in basic, translational, and pharmaceutical research. In vitro BBB models are valuable and easy to use supporting tools that can precede and complement animal and human studies. In this article, we provide a detailed review and analysis of currently available in vitro BBB models ranging from static culture systems to the most advanced flow-based and three-dimensional coculture apparatus. We also discuss recent and perspective developments in this ever expanding research field.
Copyright © 2011 Wiley Periodicals, Inc.
Conflict of interest statement
Figures

Similar articles
-
In vitro cerebrovascular modeling in the 21st century: current and prospective technologies.Pharm Res. 2014 Dec;31(12):3229-50. doi: 10.1007/s11095-014-1464-6. Epub 2014 Aug 7. Pharm Res. 2014. PMID: 25098812 Free PMC article. Review.
-
The blood-brain barrier.Handb Clin Neurol. 2016;133:39-59. doi: 10.1016/B978-0-444-63432-0.00003-7. Handb Clin Neurol. 2016. PMID: 27112670 Review.
-
Human astrocytes/astrocyte-conditioned medium and shear stress enhance the barrier properties of human brain microvascular endothelial cells.Brain Res. 2007 May 25;1147:39-50. doi: 10.1016/j.brainres.2007.02.029. Epub 2007 Feb 24. Brain Res. 2007. PMID: 17368578 Free PMC article.
-
Physiologic and anatomic characterization of the brain surface glia barrier of Drosophila.Glia. 2011 Sep;59(9):1322-40. doi: 10.1002/glia.21147. Epub 2011 Feb 23. Glia. 2011. PMID: 21351158 Free PMC article. Review.
-
Neuron-glial interactions in blood-brain barrier formation.Annu Rev Neurosci. 2007;30:235-58. doi: 10.1146/annurev.neuro.30.051606.094345. Annu Rev Neurosci. 2007. PMID: 17506642 Free PMC article. Review.
Cited by
-
Heterocellular spheroids of the neurovascular blood-brain barrier as a platform for personalized nanoneuromedicine.iScience. 2021 Feb 12;24(3):102183. doi: 10.1016/j.isci.2021.102183. eCollection 2021 Mar 19. iScience. 2021. PMID: 33718835 Free PMC article.
-
ABC Transporters at the Blood-Brain Interfaces, Their Study Models, and Drug Delivery Implications in Gliomas.Pharmaceutics. 2019 Dec 23;12(1):20. doi: 10.3390/pharmaceutics12010020. Pharmaceutics. 2019. PMID: 31878061 Free PMC article. Review.
-
Genes that Mediate Metastasis across the Blood-Brain Barrier.Trends Cancer. 2020 Aug;6(8):660-676. doi: 10.1016/j.trecan.2020.04.007. Epub 2020 May 13. Trends Cancer. 2020. PMID: 32417182 Free PMC article. Review.
-
Interactions between nanosized materials and the brain.Curr Med Chem. 2014;21(37):4200-14. doi: 10.2174/0929867321666140716100449. Curr Med Chem. 2014. PMID: 25039776 Free PMC article. Review.
-
Experimental Models to Study the Functions of the Blood-Brain Barrier.Bioengineering (Basel). 2023 Apr 25;10(5):519. doi: 10.3390/bioengineering10050519. Bioengineering (Basel). 2023. PMID: 37237588 Free PMC article. Review.
References
-
- Nag S, Kapadia A, Stewart DJ. Review: Molecular pathogenesis of blood–brain barrier breakdown in acute brain injury. Neuropathol Appl Neurobiol. 2011;37:3–23. - PubMed
-
- Traub O, Berk BC. Laminar shear stress: Mechanisms by which endothelial cells transduce an atheroprotective force. Arterioscler Thromb Vasc Biol. 1998;18:677–685. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

