Expression and regulation of chemokines in murine and human type 1 diabetes
- PMID: 22210319
- PMCID: PMC3266427
- DOI: 10.2337/db11-0853
Expression and regulation of chemokines in murine and human type 1 diabetes
Abstract
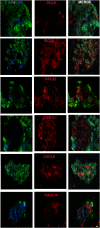
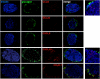
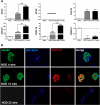
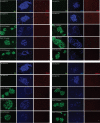
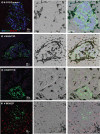
More than one-half of the ~50 human chemokines have been associated with or implicated in the pathogenesis of type 1 diabetes, yet their actual expression patterns in the islet environment of type 1 diabetic patients remain, at present, poorly defined. Here, we have integrated a human islet culture system, murine models of virus-induced and spontaneous type 1 diabetes, and the histopathological examination of pancreata from diabetic organ donors with the goal of providing a foundation for the informed selection of potential therapeutic targets within the chemokine/receptor family. Chemokine (C-C motif) ligand (CCL) 5 (CCL5), CCL8, CCL22, chemokine (C-X-C motif) ligand (CXCL) 9 (CXCL9), CXCL10, and chemokine (C-X3-C motif) ligand (CX3CL) 1 (CX3CL1) were the major chemokines transcribed (in an inducible nitric oxide synthase-dependent but not nuclear factor-κB-dependent fashion) and translated by human islet cells in response to in vitro inflammatory stimuli. CXCL10 was identified as the dominant chemokine expressed in vivo in the islet environment of prediabetic animals and type 1 diabetic patients, whereas CCL5, CCL8, CXCL9, and CX3CL1 proteins were present at lower levels in the islets of both species. Of importance, additional expression of the same chemokines in human acinar tissues emphasizes an underappreciated involvement of the exocrine pancreas in the natural course of type 1 diabetes that will require consideration for additional type 1 diabetes pathogenesis and immune intervention studies.
Figures
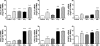
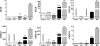
Similar articles
-
Pancreatic Alpha-Cells Contribute Together With Beta-Cells to CXCL10 Expression in Type 1 Diabetes.Front Endocrinol (Lausanne). 2020 Sep 15;11:630. doi: 10.3389/fendo.2020.00630. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33042009 Free PMC article.
-
IL-1beta and IFN-gamma induce the expression of diverse chemokines and IL-15 in human and rat pancreatic islet cells, and in islets from pre-diabetic NOD mice.Diabetologia. 2003 Feb;46(2):255-66. doi: 10.1007/s00125-002-1017-0. Epub 2003 Feb 12. Diabetologia. 2003. PMID: 12627325
-
Anti-inflammatory action of exendin-4 in human islets is enhanced by phosphodiesterase inhibitors: potential therapeutic benefits in diabetic patients.Diabetologia. 2010 Nov;53(11):2357-68. doi: 10.1007/s00125-010-1849-y. Epub 2010 Jul 16. Diabetologia. 2010. PMID: 20635178
-
Chemokine (C-X-C motif) ligand (CXCL)10 in autoimmune diseases.Autoimmun Rev. 2014 Mar;13(3):272-80. doi: 10.1016/j.autrev.2013.10.010. Epub 2013 Nov 2. Autoimmun Rev. 2014. PMID: 24189283 Review.
-
Chemokines as Drivers of the Autoimmune Destruction in Type 1 Diabetes: Opportunity for Therapeutic Intervention in Consideration of an Optimal Treatment Schedule.Front Endocrinol (Lausanne). 2020 Oct 19;11:591083. doi: 10.3389/fendo.2020.591083. eCollection 2020. Front Endocrinol (Lausanne). 2020. PMID: 33193102 Free PMC article. Review.
Cited by
-
Abnormal T Cell Frequencies, Including Cytomegalovirus-Associated Expansions, Distinguish Seroconverted Subjects at Risk for Type 1 Diabetes.Front Immunol. 2018 Oct 22;9:2332. doi: 10.3389/fimmu.2018.02332. eCollection 2018. Front Immunol. 2018. PMID: 30405601 Free PMC article.
-
Pathogenic TNF-α drives peripheral nerve inflammation in an Aire-deficient model of autoimmunity.Proc Natl Acad Sci U S A. 2022 Jan 25;119(4):e2114406119. doi: 10.1073/pnas.2114406119. Proc Natl Acad Sci U S A. 2022. PMID: 35058362 Free PMC article.
-
T Cell-Mediated Beta Cell Destruction: Autoimmunity and Alloimmunity in the Context of Type 1 Diabetes.Front Endocrinol (Lausanne). 2017 Dec 5;8:343. doi: 10.3389/fendo.2017.00343. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 29259578 Free PMC article. Review.
-
Functional redundancy of CXCR3/CXCL10 signaling in the recruitment of diabetogenic cytotoxic T lymphocytes to pancreatic islets in a virally induced autoimmune diabetes model.Diabetes. 2013 Jul;62(7):2492-9. doi: 10.2337/db12-1370. Epub 2013 Feb 22. Diabetes. 2013. PMID: 23434930 Free PMC article.
-
Encapsulation and immune protection for type 1 diabetes cell therapy.Adv Drug Deliv Rev. 2024 Apr;207:115205. doi: 10.1016/j.addr.2024.115205. Epub 2024 Feb 13. Adv Drug Deliv Rev. 2024. PMID: 38360355 Review.
References
-
- Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu Rev Immunol 2004;22:891–928 - PubMed
-
- Campbell DJ, Kim CH, Butcher EC. Chemokines in the systemic organization of immunity. Immunol Rev 2003;195:58–71 - PubMed
-
- Rotondi M, Chiovato L, Romagnani S, Serio M, Romagnani P. Role of chemokines in endocrine autoimmune diseases. Endocr Rev 2007;28:492–520 - PubMed
-
- Christen U. Chemokines as drug targets in type 1 diabetes. Endocr Metab Immune Disord Drug Targets 2007;7:7–12 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

