Directed cell invasion and migration during metastasis
- PMID: 22209238
- PMCID: PMC3320684
- DOI: 10.1016/j.ceb.2011.12.004
Directed cell invasion and migration during metastasis
Abstract
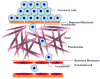
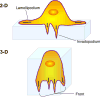
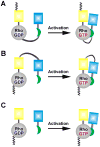
Metastasis requires tumor cell dissemination to different organs from the primary tumor. Dissemination is a complex cell motility phenomenon that requires the molecular coordination of the protrusion, chemotaxis, invasion and contractility activities of tumor cells to achieve directed cell migration. Recent studies of the spatial and temporal activities of the small GTPases have begun to elucidate how this coordination is achieved. The direct visualization of the pathways involved in actin polymerization, invasion and directed migration in dissemination competent tumor cells will help identify the molecular basis of dissemination and allow the design and testing of more specific and selective drugs to block metastasis.
Published by Elsevier Ltd.
Figures

Similar articles
-
Cell motility and cytoskeletal regulation in invasion and metastasis.J Mammary Gland Biol Neoplasia. 2007 Sep;12(2-3):143-52. doi: 10.1007/s10911-007-9046-4. J Mammary Gland Biol Neoplasia. 2007. PMID: 17557195 Review.
-
Epithelial-mesenchymal transition in tumor metastasis.Mol Oncol. 2017 Jan;11(1):28-39. doi: 10.1002/1878-0261.12017. Epub 2016 Dec 9. Mol Oncol. 2017. PMID: 28085222 Free PMC article. Review.
-
Locomotion of tumor cells as an element of invasion and metastasis.Biomed Pharmacother. 1987;41(6):337-44. Biomed Pharmacother. 1987. PMID: 3328630 Review.
-
Modes of invasion during tumour dissemination.Mol Oncol. 2017 Jan;11(1):5-27. doi: 10.1002/1878-0261.12019. Epub 2016 Dec 9. Mol Oncol. 2017. PMID: 28085224 Free PMC article. Review.
-
Actin dynamics during tumor cell dissemination.Int Rev Cell Mol Biol. 2021;360:65-98. doi: 10.1016/bs.ircmb.2020.09.004. Epub 2020 Nov 24. Int Rev Cell Mol Biol. 2021. PMID: 33962751 Free PMC article. Review.
Cited by
-
A novel NHE1-centered signaling cassette drives epidermal growth factor receptor-dependent pancreatic tumor metastasis and is a target for combination therapy.Neoplasia. 2015 Feb;17(2):155-66. doi: 10.1016/j.neo.2014.12.003. Neoplasia. 2015. PMID: 25748234 Free PMC article.
-
A Model for Direction Sensing in Dictyostelium discoideum: Ras Activity and Symmetry Breaking Driven by a Gβγ-Mediated, Gα2-Ric8 -- Dependent Signal Transduction Network.PLoS Comput Biol. 2016 May 6;12(5):e1004900. doi: 10.1371/journal.pcbi.1004900. eCollection 2016 May. PLoS Comput Biol. 2016. PMID: 27152956 Free PMC article.
-
NDRG1 regulates Filopodia-induced Colorectal Cancer invasiveness via modulating CDC42 activity.Int J Biol Sci. 2021 Apr 17;17(7):1716-1730. doi: 10.7150/ijbs.56694. eCollection 2021. Int J Biol Sci. 2021. PMID: 33994856 Free PMC article.
-
Antitumor activities of Aspiletrein A, a steroidal saponin from Aspidistra letreae, on non-small cell lung cancer cells.BMC Complement Med Ther. 2021 Mar 9;21(1):87. doi: 10.1186/s12906-021-03262-w. BMC Complement Med Ther. 2021. PMID: 33750378 Free PMC article.
-
Functions of cofilin in cell locomotion and invasion.Nat Rev Mol Cell Biol. 2013 Jul;14(7):405-15. doi: 10.1038/nrm3609. Epub 2013 Jun 19. Nat Rev Mol Cell Biol. 2013. PMID: 23778968 Free PMC article. Review.
References
-
- Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA Cancer J Clin. 2011;61:212–236. - PubMed
-
- Sporn MB. The war on cancer. Lancet. 1996;347:1377–1381. - PubMed
-
- Friedl P, Wolf K. Tumour-cell invasion and migration: diversity and escape mechanisms. Nat Rev Cancer. 2003;3:362–374. - PubMed
-
- Wang W, Goswami S, Lapidus K, Wells AL, Wyckoff JB, Sahai E, Singer RH, Segall JE, Condeelis JS. Identification and testing of a gene expression signature of invasive carcinoma cells within primary mammary tumors. Cancer research. 2004;64:8585–8594. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

