Lubricated biodegradable polymer networks for regulating nerve cell behavior and fabricating nerve conduits with a compositional gradient
- PMID: 22206477
- PMCID: PMC3544368
- DOI: 10.1021/bm201372u
Lubricated biodegradable polymer networks for regulating nerve cell behavior and fabricating nerve conduits with a compositional gradient
Abstract
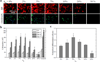
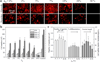
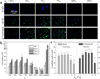
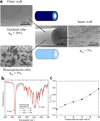
We present a method of tuning surface chemistry and nerve cell behavior by photo-cross-linking methoxy poly(ethylene glycol) monoacrylate (mPEGA) with hydrophobic, semicrystalline poly(ε-caprolactone) diacrylate (PCLDA) at various weight compositions of mPEGA (ø(m)) from 2 to 30%. Improved surface wettability is achieved with corresponding decreases in friction, water contact angle, and capability of adsorbing proteins from cell culture media because of repulsive PEG chains tethered in the network. The responses of rat Schwann cell precursor line (SpL201), rat pheochromocytoma (PC12), and E14 mouse neural progenitor cells (NPCs) to the modified surfaces are evaluated. Nonmonotonic or parabolic dependence of cell attachment, spreading, proliferation, and differentiation on ø(m) is identified for these cell types with maximal values at ø(m) of 5-7%. In addition, NPCs demonstrate enhanced neuronal differentiated lineages on the mPEGA/PCLDA network at ø(m) of 5% with intermediate wettability and surface energy. This approach lays the foundation for fabricating heterogeneous nerve conduits with a compositional gradient along the wall thickness, which are able to promote nerve cell functions within the conduit while inhibiting cell attachment on the outer wall to prevent potential fibrous tissue formation following implantation.
Figures


Similar articles
-
Photocured biodegradable polymer substrates of varying stiffness and microgroove dimensions for promoting nerve cell guidance and differentiation.Langmuir. 2012 Aug 28;28(34):12557-68. doi: 10.1021/la302868q. Epub 2012 Aug 16. Langmuir. 2012. PMID: 22857011
-
Poly(ethylene glycol)-grafted poly(propylene fumarate) networks and parabolic dependence of MC3T3 cell behavior on the network composition.Biomaterials. 2010 Jun;31(16):4457-66. doi: 10.1016/j.biomaterials.2010.02.020. Epub 2010 Mar 4. Biomaterials. 2010. PMID: 20202682
-
Promoting nerve cell functions on hydrogels grafted with poly(L-lysine).Biomacromolecules. 2012 Feb 13;13(2):342-9. doi: 10.1021/bm201763n. Epub 2012 Feb 1. Biomacromolecules. 2012. PMID: 22251248 Free PMC article.
-
Parabolic dependence of material properties and cell behavior on the composition of polymer networks via simultaneously controlling crosslinking density and crystallinity.Biomaterials. 2010 Oct;31(29):7423-34. doi: 10.1016/j.biomaterials.2010.06.028. Biomaterials. 2010. PMID: 20663551
-
The cellular response of nerve cells on poly-l-lysine coated PLGA-MWCNTs aligned nanofibers under electrical stimulation.Mater Sci Eng C Mater Biol Appl. 2018 Oct 1;91:715-726. doi: 10.1016/j.msec.2018.06.025. Epub 2018 Jun 12. Mater Sci Eng C Mater Biol Appl. 2018. PMID: 30033306
Cited by
-
Porous crosslinked polycaprolactone hydroxyapatite networks for bone tissue engineering.Tissue Eng Regen Med. 2016 Jun 9;13(3):251-260. doi: 10.1007/s13770-016-9061-x. eCollection 2016 Jun. Tissue Eng Regen Med. 2016. PMID: 30603406 Free PMC article.
-
Optimal poly(L-lysine) grafting density in hydrogels for promoting neural progenitor cell functions.Biomacromolecules. 2012 May 14;13(5):1663-74. doi: 10.1021/bm300381d. Epub 2012 May 3. Biomacromolecules. 2012. PMID: 22533450 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

