Engineering upper hinge improves stability and effector function of a human IgG1
- PMID: 22203673
- PMCID: PMC3325591
- DOI: 10.1074/jbc.M111.311811
Engineering upper hinge improves stability and effector function of a human IgG1
Abstract
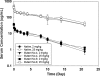
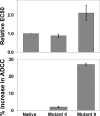
Upper hinge is vulnerable to radical attacks that result in breakage of the heavy-light chain linkage and cleavage of the hinge of an IgG1. To further explore mechanisms responsible for the radical induced hinge degradation, nine mutants were designed to determine the roles that the upper hinge Asp and His play in the radical reactions. The observation that none of these substitutions could inhibit the breakage of the heavy-light chain linkage suggests that the breakage may result from electron transfer from Cys(231) directly to the heavy-light chain linkage upon radical attacks, and implies a pathway separate from His(229)-mediated hinge cleavage. On the other hand, the substitution of His(229) with Tyr showed promising advantages over the native antibody and other substitutions in improving the stability and function of the IgG1. This substitution inhibited the hinge cleavage by 98% and suggests that the redox active nature of Tyr did not enable it to replicate the ability of His to facilitate radical induced degradation. We propose that the lower redox potential of Tyr, a residue that may be the ultimate sink for oxidizing equivalents in proteins, is responsible for the inhibition. More importantly, the substitution increased the antibody's binding to FcγRIII receptors by 2-3-fold, and improved ADCC activity by 2-fold, while maintaining a similar pharmacokinetic profile with respect to the wild type. Implications of these observations for antibody engineering and development are discussed.
Figures

Similar articles
-
Human IgG1 hinge fragmentation as the result of H2O2-mediated radical cleavage.J Biol Chem. 2009 Dec 18;284(51):35390-402. doi: 10.1074/jbc.M109.064147. J Biol Chem. 2009. PMID: 19850927 Free PMC article.
-
Breaking the light and heavy chain linkage of human immunoglobulin G1 (IgG1) by radical reactions.J Biol Chem. 2011 Jul 15;286(28):24674-84. doi: 10.1074/jbc.M111.255026. Epub 2011 May 23. J Biol Chem. 2011. PMID: 21606498 Free PMC article.
-
Relative stabilities of IgG1 and IgG4 Fab domains: influence of the light-heavy interchain disulfide bond architecture.Protein Sci. 2012 Sep;21(9):1315-22. doi: 10.1002/pro.2118. Epub 2012 Aug 9. Protein Sci. 2012. PMID: 22761163 Free PMC article.
-
Conceptual Approaches to Modulating Antibody Effector Functions and Circulation Half-Life.Front Immunol. 2019 Jun 7;10:1296. doi: 10.3389/fimmu.2019.01296. eCollection 2019. Front Immunol. 2019. PMID: 31231397 Free PMC article. Review.
-
[Heavy-chain antibodies of the Camelidae and their possible applications].Postepy Hig Med Dosw (Online). 2005 May 16;59:193-202. Postepy Hig Med Dosw (Online). 2005. PMID: 15928603 Review. Polish.
Cited by
-
Rearranging the domain order of a diabody-based IgG-like bispecific antibody enhances its antitumor activity and improves its degradation resistance and pharmacokinetics.MAbs. 2014;6(5):1243-54. doi: 10.4161/mabs.29445. Epub 2014 Oct 30. MAbs. 2014. PMID: 25517309 Free PMC article.
-
An anti-TNF-α antibody mimetic to treat ocular inflammation.Sci Rep. 2016 Nov 22;6:36905. doi: 10.1038/srep36905. Sci Rep. 2016. PMID: 27874029 Free PMC article.
-
Design, construction and in vivo functional assessment of a hinge truncated sFLT01.Gene Ther. 2023 Apr;30(3-4):347-361. doi: 10.1038/s41434-022-00362-1. Epub 2022 Sep 16. Gene Ther. 2023. PMID: 36114375
-
Effects of glycans and hinge on dynamics in the IgG1 Fc.J Biomol Struct Dyn. 2024;42(22):12571-12579. doi: 10.1080/07391102.2023.2270749. Epub 2023 Oct 28. J Biomol Struct Dyn. 2024. PMID: 37897185 Free PMC article.
-
Proteoform-Resolved FcɤRIIIa Binding Assay for Fab Glycosylated Monoclonal Antibodies Achieved by Affinity Chromatography Mass Spectrometry of Fc Moieties.Front Chem. 2019 Oct 24;7:698. doi: 10.3389/fchem.2019.00698. eCollection 2019. Front Chem. 2019. PMID: 31709228 Free PMC article.
References
-
- Nelson A. L., Dhimolea E., Reichert J. M. (2010) Development trends for human monoclonal antibody therapeutics. Nat. Rev. Drug Discov. 9, 767–774 - PubMed
-
- Carter P. J. (2006) Potent antibody therapeutics by design. Nat. Rev. Immunol. 6, 343–357 - PubMed
-
- Nelson A. L., Reichert J. M. (2009) Development trends for therapeutic antibody fragments. Nat. Biotechnol. 27, 331–337 - PubMed
-
- Beck A., Wurch T., Bailly C., Corvaia N. (2010) Strategies and challenges for the next generation of therapeutic antibodies. Nat. Rev. Immunol. 10, 345–352 - PubMed
-
- Jefferis R. (2009) Glycosylation as a strategy to improve antibody-based therapeutics. Nat. Rev. Drug Discov. 8, 226–234 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

