Metformin: multi-faceted protection against cancer
- PMID: 22203527
- PMCID: PMC3282095
- DOI: 10.18632/oncotarget.387
Metformin: multi-faceted protection against cancer
Abstract
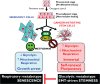
The biguanide metformin, a widely used drug for the treatment of type 2 diabetes, may exert cancer chemopreventive effects by suppressing the transformative and hyperproliferative processes that initiate carcinogenesis. Metformin's molecular targets in cancer cells (e.g., mTOR, HER2) are similar to those currently being used for directed cancer therapy. However, metformin is nontoxic and might be extremely useful for enhancing treatment efficacy of mechanism-based and biologically targeted drugs. Here, we first revisit the epidemiological, preclinical, and clinical evidence from the last 5 years showing that metformin is a promising candidate for oncology therapeutics. Second, the anticancer effects of metformin by both direct (insulin-independent) and indirect (insulin-dependent) mechanisms are discussed in terms of metformin-targeted processes and the ontogenesis of cancer stem cells (CSC), including Epithelial-to-Mesenchymal Transition (EMT) and microRNAs-regulated dedifferentiation of CSCs. Finally, we present preliminary evidence that metformin may regulate cellular senescence, an innate safeguard against cellular immortalization. There are two main lines of evidence that suggest that metformin's primary target is the immortalizing step during tumorigenesis. First, metformin activates intracellular DNA damage response checkpoints. Second, metformin attenuates the anti-senescence effects of the ATP-generating glycolytic metabotype-the Warburg effect-, which is required for self-renewal and proliferation of CSCs. If metformin therapy presents an intrinsic barrier against tumorigenesis by lowering the threshold for stress-induced senescence, metformin therapeutic strategies may be pivotal for therapeutic intervention for cancer. Current and future clinical trials will elucidate whether metformin has the potential to be used in preventive and treatment settings as an adjuvant to current cancer therapeutics.
Conflict of interest statement
None to declare.
Figures



Similar articles
-
Metformin and the ATM DNA damage response (DDR): accelerating the onset of stress-induced senescence to boost protection against cancer.Aging (Albany NY). 2011 Nov;3(11):1063-77. doi: 10.18632/aging.100407. Aging (Albany NY). 2011. PMID: 22170748 Free PMC article. Review.
-
Pharmacologic mechanisms underlying antidiabetic drug metformin's chemopreventive effect against colorectal cancer.Eur J Pharmacol. 2021 Apr 15;897:173956. doi: 10.1016/j.ejphar.2021.173956. Epub 2021 Feb 19. Eur J Pharmacol. 2021. PMID: 33617821 Review.
-
Metformin against TGFβ-induced epithelial-to-mesenchymal transition (EMT): from cancer stem cells to aging-associated fibrosis.Cell Cycle. 2010 Nov 15;9(22):4461-8. doi: 10.4161/cc.9.22.14048. Epub 2010 Nov 15. Cell Cycle. 2010. PMID: 21088486
-
Metformin lowers the threshold for stress-induced senescence: a role for the microRNA-200 family and miR-205.Cell Cycle. 2012 Mar 15;11(6):1235-46. doi: 10.4161/cc.11.6.19665. Epub 2012 Mar 15. Cell Cycle. 2012. PMID: 22356767
-
Repositioning chloroquine and metformin to eliminate cancer stem cell traits in pre-malignant lesions.Drug Resist Updat. 2011 Aug-Oct;14(4-5):212-23. doi: 10.1016/j.drup.2011.04.003. Epub 2011 May 19. Drug Resist Updat. 2011. PMID: 21600837 Free PMC article.
Cited by
-
Metformin and the promise of geroprotection.Indian J Endocrinol Metab. 2012 Jul;16(4):496-8. doi: 10.4103/2230-8210.97991. Indian J Endocrinol Metab. 2012. PMID: 22837902 Free PMC article. No abstract available.
-
Ras/Raf/MEK/ERK and PI3K/PTEN/Akt/mTOR cascade inhibitors: how mutations can result in therapy resistance and how to overcome resistance.Oncotarget. 2012 Oct;3(10):1068-111. doi: 10.18632/oncotarget.659. Oncotarget. 2012. PMID: 23085539 Free PMC article. Review.
-
Low concentrations of metformin selectively inhibit CD133⁺ cell proliferation in pancreatic cancer and have anticancer action.PLoS One. 2013 May 8;8(5):e63969. doi: 10.1371/journal.pone.0063969. Print 2013. PLoS One. 2013. PMID: 23667692 Free PMC article.
-
Metformin sensitizes anticancer effect of dasatinib in head and neck squamous cell carcinoma cells through AMPK-dependent ER stress.Oncotarget. 2014 Jan 15;5(1):298-308. doi: 10.18632/oncotarget.1628. Oncotarget. 2014. PMID: 24457597 Free PMC article.
-
Metformin Is Associated With Slightly Reduced Risk of Colorectal Cancer and Moderate Survival Benefits in Diabetes Mellitus: A Meta-Analysis.Medicine (Baltimore). 2016 Feb;95(7):e2749. doi: 10.1097/MD.0000000000002749. Medicine (Baltimore). 2016. PMID: 26886616 Free PMC article. Review.
References
-
- Pollak M. Insulin and insulin-like growth factor signalling in neoplasia. Nat Rev Cancer. 2008;8:915–28. - PubMed
-
- Larsson SC, Mantzoros CS, Wolk A. Diabetes mellitus and risk of breast cancer: a meta-analysis. Int J Cancer. 2007;121:856–62. - PubMed
-
- Smith U, Gale EM. Cancer and diabetes: are we ready for prime time? Diabetologia. 2010;53:1541–4. - PubMed
-
- Vigneri P, Frasca F, Sciacca L, Pandini G, Vigneri R. Diabetes and cancer. Endocr Relat Cancer. 2009;16:1103–23. - PubMed
-
- Bonovas S, Filioussi K, Tsantes A. Diabetes mellitus and risk of prostate cancer: a meta-analysis. Diabetologia. 2004;47:1071–1078. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

