Phospholipase PLA2G7, associated with aggressive prostate cancer, promotes prostate cancer cell migration and invasion and is inhibited by statins
- PMID: 22202492
- PMCID: PMC3282076
- DOI: 10.18632/oncotarget.397
Phospholipase PLA2G7, associated with aggressive prostate cancer, promotes prostate cancer cell migration and invasion and is inhibited by statins
Abstract
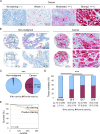
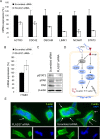
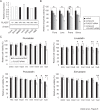
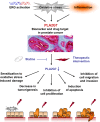
Prostate cancer is the second leading cause of cancer mortality in men in developed countries. Due to the heterogeneous nature of the disease, design of novel personalized treatments is required to achieve efficient therapeutic responses. We have recently identified phospholipase 2 group VII (PLA2G7) as a potential drug target especially in ERG oncogene positive prostate cancers. Here, the expression profile of PLA2G7 was studied in 1137 prostate cancer and 409 adjacent non-malignant prostate tissues using immunohistochemistry to validate its biomarker potential and putative association with disease progression. In order to reveal the molecular alterations induced by PLA2G7 impairment, lipidomic and gene expression profiling was performed in response to PLA2G7 silencing in cultured prostate cancer cells. Moreover, the antineoplastic effect of statins combined with PLA2G7 impairment was studied in prostate cancer cells to evaluate the potential of repositioning of in vivo compatible drugs developed for other indications towards anti-cancer purposes. The results indicated that PLA2G7 is a cancer-selective biomarker in 50 % of prostate cancers and associates with aggressive disease. The alterations induced by PLA2G7 silencing highlighted the potential of PLA2G7 inhibition as an anti-proliferative, pro-apoptotic and anti-migratorial therapeutic approach in prostate cancer. Moreover, the anti-proliferative effect of PLA2G7 silencing was potentiated by lipid-lowering statins in prostate cancer cells. Taken together, our results support the potential of PLA2G7 as a biomarker and a drug target in prostate cancer and present a rationale for combining PLA2G7 inhibition with the use of statins in prostate cancer management.
Figures


Similar articles
-
Arachidonic acid pathway members PLA2G7, HPGD, EPHX2, and CYP4F8 identified as putative novel therapeutic targets in prostate cancer.Am J Pathol. 2011 Feb;178(2):525-36. doi: 10.1016/j.ajpath.2010.10.002. Am J Pathol. 2011. PMID: 21281786 Free PMC article.
-
Characterization of transcriptional changes in ERG rearrangement-positive prostate cancer identifies the regulation of metabolic sensors such as neuropeptide Y.PLoS One. 2013;8(2):e55207. doi: 10.1371/journal.pone.0055207. Epub 2013 Feb 4. PLoS One. 2013. PMID: 23390522 Free PMC article.
-
Validation of Novel Biomarkers for Prostate Cancer Progression by the Combination of Bioinformatics, Clinical and Functional Studies.PLoS One. 2016 May 19;11(5):e0155901. doi: 10.1371/journal.pone.0155901. eCollection 2016. PLoS One. 2016. PMID: 27196083 Free PMC article.
-
Biologic and epidemiologic evidence assessing if statins prevent prostate cancer.Can J Urol. 2017 Dec;24(6):9081-9088. Can J Urol. 2017. PMID: 29260632 Review.
-
Advances in ovarian cancer treatment using a combination of statins with other drugs.Front Pharmacol. 2023 Jan 4;13:1048484. doi: 10.3389/fphar.2022.1048484. eCollection 2022. Front Pharmacol. 2023. PMID: 36686716 Free PMC article. Review.
Cited by
-
Proteomics biomarker discovery for individualized prevention of familial pancreatic cancer using statistical learning.PLoS One. 2023 Jan 26;18(1):e0280399. doi: 10.1371/journal.pone.0280399. eCollection 2023. PLoS One. 2023. PMID: 36701413 Free PMC article.
-
Phospholipase PLA2G7 is complementary to GPX4 in mitigating punicic-acid-induced ferroptosis in prostate cancer cells.iScience. 2024 Apr 18;27(5):109774. doi: 10.1016/j.isci.2024.109774. eCollection 2024 May 17. iScience. 2024. PMID: 38711443 Free PMC article.
-
Aggressive rat prostate tumors reprogram the benign parts of the prostate and regional lymph nodes prior to metastasis.PLoS One. 2017 May 4;12(5):e0176679. doi: 10.1371/journal.pone.0176679. eCollection 2017. PLoS One. 2017. PMID: 28472073 Free PMC article.
-
Association of six CpG-SNPs in the inflammation-related genes with coronary heart disease.Hum Genomics. 2016 Jul 25;10 Suppl 2(Suppl 2):21. doi: 10.1186/s40246-016-0067-1. Hum Genomics. 2016. PMID: 27461004 Free PMC article.
-
Statin-induced depletion of geranylgeranyl pyrophosphate inhibits cell proliferation by a novel pathway of Skp2 degradation.Oncotarget. 2015 Feb 20;6(5):2889-902. doi: 10.18632/oncotarget.3068. Oncotarget. 2015. PMID: 25605247 Free PMC article.
References
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Tomlins SA, Rhodes DR, Perner S, Dhanasekaran SM, Mehra R, Sun XW, Varambally S, Cao X, Tchinda J, Kuefer R, Lee C, Montie JE, Shah RB, Pienta KJ, Rubin MA, Chinnaiyan AM. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science. 2005;310:644–8. - PubMed
-
- Gupta S, Iljin K, Sara H, Mpindi JP, Mirtti T, Vainio P, Rantala J, Alanen K, Nees M, Kallioniemi O. FZD4 as a mediator of ERG oncogene-induced WNT signaling and epithelial-to-mesenchymal transition in human prostate cancer cells. Cancer Res. 2010;70:6735–45. - PubMed
-
- Iljin K, Wolf M, Edgren H, Gupta S, Kilpinen S, Skotheim RI, Peltola M, Smit F, Verhaegh G, Schalken J, Nees M, Kallioniemi O. TMPRSS2 fusions with oncogenic ETS factors in prostate cancer involve unbalanced genomic rearrangements and are associated with HDAC1 and epigenetic reprogramming. Cancer Res. 2006;66:10242–6. - PubMed
-
- Sun C, Dobi A, Mohamed A, Li H, Thangapazham RL, Furusato B, Shaheduzzaman S, Tan SH, Vaidyanathan G, Whitman E, Hawksworth DJ, Chen Y, Nau M, Patel V, Vahey M, Gutkind JS, et al. TMPRSS2-ERG fusion, a common genomic alteration in prostate cancer activates C-MYC and abrogates prostate epithelial differentiation. Oncogene. 2008;27:5348–53. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

