The membrane-bound mucin Muc1 regulates T helper 17-cell responses and colitis in mice
- PMID: 22202458
- PMCID: PMC3441148
- DOI: 10.1053/j.gastro.2011.12.036
The membrane-bound mucin Muc1 regulates T helper 17-cell responses and colitis in mice
Abstract
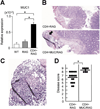
Background & aims: T helper (Th) 17 cells produce the effector cytokine interleukin (IL)-17, along with IL-22, which stimulates colonic epithelial cells to produce a membrane-bound mucin, Muc1. Muc1 is a component of the colonic mucus, which functions as a lubricant and a physiologic barrier between luminal contents and mucosal surface. The gene MUC1 has been associated with susceptibility to inflammatory bowel disease; we investigated the role of Muc1 in development of colitis in mice.
Methods: Muc1 and RAG1 were disrupted in mice (Muc/RAG double knockout mice); Th1-mediated colitis was induced by intravenous injection of CD4(+)CD45RB(high) T cells. We also studied Th2-mediated colitis using mice with disruptions in Muc1 and T-cell receptor α chain (Muc/TCR double knockout mice).
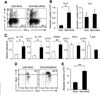
Results: Muc1 deficiency led to the development of more severe forms of Th1- and Th2-induced colitis than controls. Loss of Muc1 increased colonic permeability and the Th17-cell, but not Th2 or Th1 cell, response in the inflamed colon. Loss of Muc1 also promoted expansion of an innate lymphoid cell population (Lin(-) ckit(-) Thy1(+) Sca1(+)) that produces IL-17. The expansion of Th17 adaptive immune cells and innate lymphoid cells required the commensal microbiota.
Conclusions: Muc1, which is up-regulated by Th17 signaling, functions in a negative feedback pathway that prevents an excessive Th17 cell response in inflamed colons of mice. Disruption of this negative feedback pathway, perhaps by variants in Muc1, might contribute to inflammatory bowel disease in patients.
Copyright © 2012 AGA Institute. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflicts of interest:
The authors disclose no conflicts.
Figures
Similar articles
-
Innate PI3K p110δ regulates Th1/Th17 development and microbiota-dependent colitis.J Immunol. 2014 Apr 15;192(8):3958-68. doi: 10.4049/jimmunol.1301533. Epub 2014 Mar 14. J Immunol. 2014. PMID: 24634494 Free PMC article.
-
Glucocorticoid-induced tumor necrosis factor receptor family-related protein regulates CD4(+)T cell-mediated colitis in mice.Gastroenterology. 2012 Mar;142(3):582-591.e8. doi: 10.1053/j.gastro.2011.11.031. Epub 2011 Dec 6. Gastroenterology. 2012. PMID: 22155173 Free PMC article.
-
Effects of hypoxic exposure on immune responses of intestinal mucosa to Citrobacter colitis in mice.Biomed Pharmacother. 2020 Sep;129:110477. doi: 10.1016/j.biopha.2020.110477. Epub 2020 Jul 6. Biomed Pharmacother. 2020. PMID: 32768962
-
Development, validation and implementation of an in vitro model for the study of metabolic and immune function in normal and inflamed human colonic epithelium.Dan Med J. 2015 Jan;62(1):B4973. Dan Med J. 2015. PMID: 25557335 Review.
-
Synergy of IL-23 and Th17 cytokines: new light on inflammatory bowel disease.Neurochem Res. 2010 Jun;35(6):940-6. doi: 10.1007/s11064-009-0091-9. Epub 2009 Nov 14. Neurochem Res. 2010. PMID: 19915978 Free PMC article. Review.
Cited by
-
Nanoparticle curcumin ameliorates experimental colitis via modulation of gut microbiota and induction of regulatory T cells.PLoS One. 2017 Oct 6;12(10):e0185999. doi: 10.1371/journal.pone.0185999. eCollection 2017. PLoS One. 2017. PMID: 28985227 Free PMC article.
-
Intestinal Inflammation Induced by Soybean Meal Ingestion Increases Intestinal Permeability and Neutrophil Turnover Independently of Microbiota in Zebrafish.Front Immunol. 2020 Jul 24;11:1330. doi: 10.3389/fimmu.2020.01330. eCollection 2020. Front Immunol. 2020. PMID: 32793187 Free PMC article.
-
IL-21 induces IL-22 production in CD4+ T cells.Nat Commun. 2014 May 6;5:3753. doi: 10.1038/ncomms4753. Nat Commun. 2014. PMID: 24796415 Free PMC article.
-
Mammalian Neuraminidases in Immune-Mediated Diseases: Mucins and Beyond.Front Immunol. 2022 Apr 11;13:883079. doi: 10.3389/fimmu.2022.883079. eCollection 2022. Front Immunol. 2022. PMID: 35479093 Free PMC article.
-
MUC1 Mucin: A Putative Regulatory (Checkpoint) Molecule of T Cells.Front Immunol. 2018 Oct 22;9:2391. doi: 10.3389/fimmu.2018.02391. eCollection 2018. Front Immunol. 2018. PMID: 30405607 Free PMC article. Review.
References
-
- Xavier RJ, Podolsky DK. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- RC1 DK086242/DK/NIDDK NIH HHS/United States
- R01 AI081807-01A2/AI/NIAID NIH HHS/United States
- R01 DK080070/DK/NIDDK NIH HHS/United States
- RC1 DK086242-01/DK/NIDDK NIH HHS/United States
- R01 AI081807/AI/NIAID NIH HHS/United States
- RC1DK086242/DK/NIDDK NIH HHS/United States
- RC1 DK086242-02/DK/NIDDK NIH HHS/United States
- R01AI081807/AI/NIAID NIH HHS/United States
- R01 DK082427/DK/NIDDK NIH HHS/United States
- R01DK080070/DK/NIDDK NIH HHS/United States
- P30 DK043351/DK/NIDDK NIH HHS/United States
- R01DK082427/DK/NIDDK NIH HHS/United States
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

