Nef decreases HIV-1 sensitivity to neutralizing antibodies that target the membrane-proximal external region of TMgp41
- PMID: 22194689
- PMCID: PMC3240605
- DOI: 10.1371/journal.ppat.1002442
Nef decreases HIV-1 sensitivity to neutralizing antibodies that target the membrane-proximal external region of TMgp41
Abstract
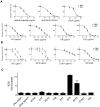
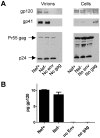
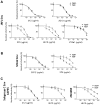
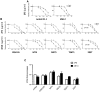
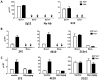
Primate lentivirus nef is required for sustained virus replication in vivo and accelerated progression to AIDS. While exploring the mechanism by which Nef increases the infectivity of cell-free virions, we investigated a functional link between Nef and Env. Since we failed to detect an effect of Nef on the quantity of virion-associated Env, we searched for qualitative changes by examining whether Nef alters HIV-1 sensitivity to agents that target distinct features of Env. Nef conferred as much as 50-fold resistance to 2F5 and 4E10, two potent neutralizing monoclonal antibodies (nAbs) that target the membrane proximal external region (MPER) of TMgp41. In contrast, Nef had no effect on HIV-1 neutralization by MPER-specific nAb Z13e1, by the peptide inhibitor T20, nor by a panel of nAbs and other reagents targeting gp120. Resistance to neutralization by 2F5 and 4E10 was observed with Nef from a diverse range of HIV-1 and SIV isolates, as well as with HIV-1 virions bearing Env from CCR5- and CXCR4-tropic viruses, clade B and C viruses, or primary isolates. Functional analysis of a panel of Nef mutants revealed that this activity requires Nef myristoylation but that it is genetically separable from other Nef functions such as the ability to enhance virus infectivity and to downregulate CD4. Glycosylated-Gag from MoMLV substituted for Nef in conferring resistance to 2F5 and 4E10, indicating that this activity is conserved in a retrovirus that does not encode Nef. Given the reported membrane-dependence of MPER-recognition by 2F5 and 4E10, in contrast to the membrane-independence of Z13e1, the data here is consistent with a model in which Nef alters MPER recognition in the context of the virion membrane. Indeed, Nef and Glycosylated-Gag decreased the efficiency of virion capture by 2F5 and 4E10, but not by other nAbs. These studies demonstrate that Nef protects lentiviruses from one of the most broadly-acting classes of neutralizing antibodies. This newly discovered activity for Nef has important implications for anti-HIV-1 immunity and AIDS pathogenesis.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
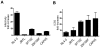

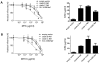
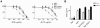
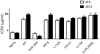
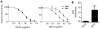

Similar articles
-
HIV type 1 Env precursor cleavage state affects recognition by both neutralizing and nonneutralizing gp41 antibodies.AIDS Res Hum Retroviruses. 2011 Aug;27(8):877-87. doi: 10.1089/AID.2010.0281. Epub 2011 Jan 19. AIDS Res Hum Retroviruses. 2011. PMID: 21158699 Free PMC article.
-
Direct antibody access to the HIV-1 membrane-proximal external region positively correlates with neutralization sensitivity.J Virol. 2011 Aug;85(16):8217-26. doi: 10.1128/JVI.00756-11. Epub 2011 Jun 8. J Virol. 2011. PMID: 21653673 Free PMC article.
-
Anti-human immunodeficiency virus type 1 (HIV-1) antibodies 2F5 and 4E10 require surprisingly few crucial residues in the membrane-proximal external region of glycoprotein gp41 to neutralize HIV-1.J Virol. 2005 Jan;79(2):1252-61. doi: 10.1128/JVI.79.2.1252-1261.2005. J Virol. 2005. PMID: 15613352 Free PMC article.
-
Antigp41 membrane proximal external region antibodies and the art of using the membrane for neutralization.Curr Opin HIV AIDS. 2017 May;12(3):250-256. doi: 10.1097/COH.0000000000000364. Curr Opin HIV AIDS. 2017. PMID: 28422789 Review.
-
Neutralizing Antibodies Targeting HIV-1 gp41.Viruses. 2020 Oct 23;12(11):1210. doi: 10.3390/v12111210. Viruses. 2020. PMID: 33114242 Free PMC article. Review.
Cited by
-
Structure-guided alterations of the gp41-directed HIV-1 broadly neutralizing antibody 2F5 reveal new properties regarding its neutralizing function.PLoS Pathog. 2012;8(7):e1002806. doi: 10.1371/journal.ppat.1002806. Epub 2012 Jul 19. PLoS Pathog. 2012. PMID: 22829767 Free PMC article.
-
Antiviral HIV-1 SERINC restriction factors disrupt virus membrane asymmetry.Nat Commun. 2023 Jul 20;14(1):4368. doi: 10.1038/s41467-023-39262-2. Nat Commun. 2023. PMID: 37474505 Free PMC article.
-
Identification of a cluster of HIV-1 controllers infected with low replicating viruses.PLoS One. 2013 Oct 30;8(10):e77663. doi: 10.1371/journal.pone.0077663. eCollection 2013. PLoS One. 2013. PMID: 24204910 Free PMC article.
-
Comparative proteomic analysis of HIV-1 particles reveals a role for Ezrin and EHD4 in the Nef-dependent increase of virus infectivity.J Virol. 2013 Apr;87(7):3729-40. doi: 10.1128/JVI.02477-12. Epub 2013 Jan 16. J Virol. 2013. PMID: 23325686 Free PMC article.
-
The frantic play of the concealed HIV envelope cytoplasmic tail.Retrovirology. 2013 May 24;10:54. doi: 10.1186/1742-4690-10-54. Retrovirology. 2013. PMID: 23705972 Free PMC article. Review.
References
-
- Kestler HW, 3rd, Ringler DJ, Mori K, Panicali DL, Sehgal PK, et al. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. - PubMed
-
- Deacon NJ, Tsykin A, Solomon A, Smith K, Ludford-Menting M, et al. Genomic structure of an attenuated quasi species of HIV-1 from a blood transfusion donor and recipients. Science. 1995;270:988–991. - PubMed
-
- Kirchhoff F, Greenough TC, Brettler DB, Sullivan JL, Desrosiers RC. Brief report: absence of intact nef sequences in a long-term survivor with nonprogressive HIV-1 infection. N Engl J Med. 1995;332:228–232. - PubMed
-
- Garcia JV, Miller AD. Serine phosphorylation-independent downregulation of cell-surface CD4 by nef. Nature. 1991;350:508–511. - PubMed
-
- Aiken C, Konner J, Landau NR, Lenburg ME, Trono D. Nef induces CD4 endocytosis: requirement for a critical dileucine motif in the membrane-proximal CD4 cytoplasmic domain. Cell. 1994;76:853–864. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

