Hypoxic activation of ATR and the suppression of the initiation of DNA replication through cdc6 degradation
- PMID: 22179839
- PMCID: PMC3310967
- DOI: 10.1038/onc.2011.585
Hypoxic activation of ATR and the suppression of the initiation of DNA replication through cdc6 degradation
Abstract
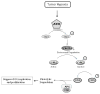
Many severely hypoxic cells fail to initiate DNA replication, but the mechanism underlying this observation is unknown. Specifically, although the ataxia-telangiectasia-rad3 related (ATR) kinase has been shown to be activated in hypoxic cells, several studies have not been able to document down-stream consequences of ATR activation in these cells. By clearly defining the DNA replication initiation checkpoint in hypoxic cells, we now demonstrate that ATR is responsible for activating this checkpoint. We show that the hypoxic activation of ATR leads to the phosphorylation-dependent degradation of the cdc25a phosphatase. Downregulation of cdc25a protein by ATR in hypoxic cells decreases CDK2 phosphorylation and activity, which results in the degradation of cdc6 by APC/C(Cdh1). These events do not occur in hypoxic cells when ATR is depleted, and the initiation of DNA replication is maintained. We therefore present a novel mechanism of cdc6 regulation in which ATR can have a central role in inhibiting the initiation of DNA replication by the regulation of cdc6 by APC/C(Cdh1). This model provides insight into the biology and therapy of hypoxic tumors.
Conflict of interest statement
The authors declare no conflicts of interest
Figures
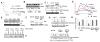
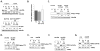


Similar articles
-
Deregulated Cdc6 inhibits DNA replication and suppresses Cdc7-mediated phosphorylation of Mcm2-7 complex.Nucleic Acids Res. 2010 Sep;38(16):5409-18. doi: 10.1093/nar/gkq262. Epub 2010 Apr 26. Nucleic Acids Res. 2010. PMID: 20421204 Free PMC article.
-
Activation of a novel ataxia-telangiectasia mutated and Rad3 related/checkpoint kinase 1-dependent prometaphase checkpoint in cancer cells by diallyl trisulfide, a promising cancer chemopreventive constituent of processed garlic.Mol Cancer Ther. 2007 Apr;6(4):1249-61. doi: 10.1158/1535-7163.MCT-06-0477. Epub 2007 Apr 3. Mol Cancer Ther. 2007. PMID: 17406033
-
CDC6 interaction with ATR regulates activation of a replication checkpoint in higher eukaryotic cells.J Cell Sci. 2010 Jan 15;123(Pt 2):225-35. doi: 10.1242/jcs.058693. J Cell Sci. 2010. PMID: 20048340
-
TopBP1 and DNA polymerase alpha-mediated recruitment of the 9-1-1 complex to stalled replication forks: implications for a replication restart-based mechanism for ATR checkpoint activation.Cell Cycle. 2009 Sep 15;8(18):2877-84. doi: 10.4161/cc.8.18.9485. Epub 2009 Sep 9. Cell Cycle. 2009. PMID: 19652550 Review.
-
Cdc25A phosphatase: combinatorial phosphorylation, ubiquitylation and proteolysis.Oncogene. 2004 Mar 15;23(11):2050-6. doi: 10.1038/sj.onc.1207394. Oncogene. 2004. PMID: 15021892 Review.
Cited by
-
Targeting radiation-resistant hypoxic tumour cells through ATR inhibition.Br J Cancer. 2012 Jul 10;107(2):291-9. doi: 10.1038/bjc.2012.265. Epub 2012 Jun 19. Br J Cancer. 2012. PMID: 22713662 Free PMC article.
-
Epigenetic Reprogramming of Kaposi's Sarcoma-Associated Herpesvirus during Hypoxic Reactivation.Cancers (Basel). 2022 Nov 2;14(21):5396. doi: 10.3390/cancers14215396. Cancers (Basel). 2022. PMID: 36358814 Free PMC article.
-
Claspin-Dependent and -Independent Chk1 Activation by a Panel of Biological Stresses.Biomolecules. 2023 Jan 7;13(1):125. doi: 10.3390/biom13010125. Biomolecules. 2023. PMID: 36671510 Free PMC article.
-
Identification and characterization of small molecules that inhibit nonsense-mediated RNA decay and suppress nonsense p53 mutations.Cancer Res. 2014 Jun 1;74(11):3104-13. doi: 10.1158/0008-5472.CAN-13-2235. Epub 2014 Mar 24. Cancer Res. 2014. PMID: 24662918 Free PMC article.
-
HIF1α-Regulated Expression of the Fatty Acid Binding Protein Family Is Important for Hypoxic Reactivation of Kaposi's Sarcoma-Associated Herpesvirus.J Virol. 2021 May 24;95(12):e02063-20. doi: 10.1128/JVI.02063-20. Print 2021 May 24. J Virol. 2021. PMID: 33789996 Free PMC article.
References
-
- Benmaamar R, Pagano M. Involvement of the SCF complex in the control of Cdh1 degradation in S-phase. Cell Cycle. 2005;4:1230–1232. - PubMed
-
- Borlado LR, Mendez J. CDC6: from DNA replication to cell cycle checkpoints and oncogenesis. Carcinogenesis. 2008;29:237–243. - PubMed
-
- Busino L, Donzelli M, Chiesa M, Guardavaccaro D, Ganoth D, Dorrello NV, et al. Degradation of Cdc25A by beta-TrCP during S phase and in response to DNA damage. Nature. 2003;426:87–91. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

