N-methyl-D-aspartate receptor mechanosensitivity is governed by C terminus of NR2B subunit
- PMID: 22179603
- PMCID: PMC3281702
- DOI: 10.1074/jbc.M111.253740
N-methyl-D-aspartate receptor mechanosensitivity is governed by C terminus of NR2B subunit
Abstract
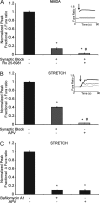
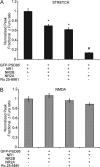
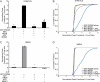
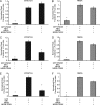
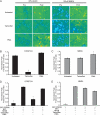
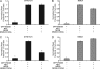
N-methyl-D-aspartate receptors (NMDARs), critical mediators of both physiologic and pathologic neurological signaling, have previously been shown to be sensitive to mechanical stretch through the loss of its native Mg(2+) block. However, the regulation of this mechanosensitivity has yet to be further explored. Furthermore, as it has become apparent that NMDAR-mediated signaling is dependent on specific NMDAR subtypes, as governed by the identity of the NR2 subunit, a crucial unanswered question is the role of subunit composition in observed NMDAR mechanosensitivity. Here, we used a recombinant system to assess the mechanosensitivity of specific subtypes and demonstrate that the mechanosensitive property is uniquely governed by the NR2B subunit. NR1/NR2B NMDARs displayed significant stretch sensitivity, whereas NR1/NR2A NMDARs did not respond to stretch. Furthermore, NR2B mechanosensitivity was regulated by PKC activity, because PKC inhibition reduced stretch responses in transfected HEK 293 cells and primary cortical neurons. Finally, using NR2B point mutations, we identified a PKC phosphorylation site, Ser-1323 on NR2B, as a unique critical regulator of stretch sensitivity. These data suggest that the selective mechanosensitivity of NR2B can significantly impact neuronal response to traumatic brain injury and illustrate that the mechanical tone of the neuron can be dynamically regulated by PKC activity.
Figures
Similar articles
-
Traumatic mechanical injury to the hippocampus in vitro causes regional caspase-3 and calpain activation that is influenced by NMDA receptor subunit composition.Neurobiol Dis. 2006 Apr;22(1):165-76. doi: 10.1016/j.nbd.2005.10.011. Epub 2005 Dec 13. Neurobiol Dis. 2006. PMID: 16356733
-
Effects of acute and chronic ethanol exposure on heteromeric N-methyl-D-aspartate receptors expressed in HEK 293 cells.J Neurochem. 1997 Dec;69(6):2345-54. doi: 10.1046/j.1471-4159.1997.69062345.x. J Neurochem. 1997. PMID: 9375665
-
Protein kinase C enhances glycine-insensitive desensitization of NMDA receptors independently of previously identified protein kinase C sites.J Neurochem. 2006 Mar;96(6):1509-18. doi: 10.1111/j.1471-4159.2006.03651.x. Epub 2006 Jan 17. J Neurochem. 2006. PMID: 16417568
-
NMDA receptor function: subunit composition versus spatial distribution.Cell Tissue Res. 2006 Nov;326(2):439-46. doi: 10.1007/s00441-006-0273-6. Epub 2006 Jul 22. Cell Tissue Res. 2006. PMID: 16862427 Review.
-
A Review on Chronic Pain in Rheumatoid Arthritis: A Focus on Activation of NR2B Subunit of N-Methyl-D-Aspartate Receptors.Malays J Med Sci. 2020 Feb;27(1):6-21. doi: 10.21315/mjms2020.27.1.2. Epub 2020 Feb 27. Malays J Med Sci. 2020. PMID: 32158341 Free PMC article. Review.
Cited by
-
N-Methyl-D-Aspartate Receptor Signaling and Function in Cardiovascular Tissues.J Cardiovasc Pharmacol. 2016 Aug;68(2):97-105. doi: 10.1097/FJC.0000000000000398. J Cardiovasc Pharmacol. 2016. PMID: 27046337 Free PMC article. Review.
-
Distribution of extrasynaptic NMDA receptors on neurons.ScientificWorldJournal. 2012;2012:267120. doi: 10.1100/2012/267120. Epub 2012 Apr 30. ScientificWorldJournal. 2012. PMID: 22654580 Free PMC article. Review.
-
A multilayer network model of neuron-astrocyte populations in vitro reveals mGluR5 inhibition is protective following traumatic injury.Netw Neurosci. 2022 Jun 1;6(2):499-527. doi: 10.1162/netn_a_00227. eCollection 2022 Jun. Netw Neurosci. 2022. PMID: 35733423 Free PMC article.
-
Viral vector-mediated upregulation of serine racemase expression in medial prefrontal cortex improves learning and synaptic function in middle age rats.Aging (Albany NY). 2023 Apr 12;15(7):2433-2449. doi: 10.18632/aging.204652. Epub 2023 Apr 12. Aging (Albany NY). 2023. PMID: 37052995 Free PMC article.
-
Memantine improves outcomes after repetitive traumatic brain injury.Behav Brain Res. 2018 Mar 15;340:195-204. doi: 10.1016/j.bbr.2017.04.017. Epub 2017 Apr 13. Behav Brain Res. 2018. PMID: 28412305 Free PMC article.
References
-
- Hardingham G. E., Fukunaga Y., Bading H. (2002) Extrasynaptic NMDARs oppose synaptic NMDARs by triggering CREB shut-off and cell death pathways. Nat. Neurosci. 5, 405–414 - PubMed
-
- Hetman M., Kharebava G. (2006) Survival signaling pathways activated by NMDA receptors. Curr. Top Med. Chem. 6, 787–799 - PubMed
-
- Ikonomidou C., Bosch F., Miksa M., Bittigau P., Vöckler J., Dikranian K., Tenkova T. I., Stefovska V., Turski L., Olney J. W. (1999) Blockade of NMDA receptors and apoptotic neurodegeneration in the developing brain. Science 283, 70–74 - PubMed
-
- Cull-Candy S. G., Leszkiewicz D. N. (2004) Role of distinct NMDA receptor subtypes at central synapses. Sci. STKE 2004, re16. - PubMed
-
- Dingledine R., Borges K., Bowie D., Traynelis S. F. (1999) The glutamate receptor ion channels. Pharmacol. Rev. 51, 7–61 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

