Immunogenicity and efficacy of chimeric dengue vaccine (DENVax) formulations in interferon-deficient AG129 mice
- PMID: 22178727
- PMCID: PMC4592107
- DOI: 10.1016/j.vaccine.2011.11.072
Immunogenicity and efficacy of chimeric dengue vaccine (DENVax) formulations in interferon-deficient AG129 mice
Abstract
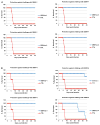
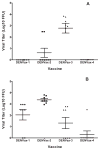
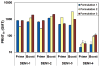
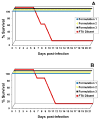
Formulations of chimeric dengue vaccine (DENVax) viruses containing the pre-membrane (prM) and envelope (E) genes of serotypes 1-4 expressed in the context of the attenuated DENV-2 PDK-53 genome were tested for safety, immunogenicity and efficacy in interferon receptor knock-out mice (AG129). Monovalent formulations were safe and elicited robust neutralizing antibody responses to the homologous virus and only limited cross-reactivity to other serotypes. A single dose of monovalent DENVax-1, -2, or -3 vaccine provided eighty or greater percent protection against both wild-type (wt) DENV-1 (Mochizuki strain) and DENV-2 (New Guinea C strain) challenge viruses. A single dose of monovalent DENVax-4 also provided complete protection against wt DENV-1 challenge and significantly increased the survival times after challenge with wt DENV-2. In studies using tetravalent mixtures, DENVax ratios were identified that: (i) caused limited viremia, (ii) induced serotype-specific neutralizing antibodies to all four DENV serotypes with different hierarchies, and (iii) conferred full protection against clinical signs of disease following challenge with either wt DENV-1 or DENV-2 viruses. Overall, these data highlight the immunogenic profile of DENVax, a novel candidate tetravalent dengue vaccine and the advantage of sharing a common attenuated genomic backbone among the DENVax monovalent vaccines that confer protection against homologous or heterologous virus challenge.
Copyright © 2011 Elsevier Ltd. All rights reserved.
Figures
Similar articles
-
Safety and immunogenicity of a recombinant live attenuated tetravalent dengue vaccine (DENVax) in flavivirus-naive healthy adults in Colombia: a randomised, placebo-controlled, phase 1 study.Lancet Infect Dis. 2014 Sep;14(9):830-8. doi: 10.1016/S1473-3099(14)70811-4. Epub 2014 Jul 23. Lancet Infect Dis. 2014. PMID: 25087476 Free PMC article. Clinical Trial.
-
Development of DENVax: a chimeric dengue-2 PDK-53-based tetravalent vaccine for protection against dengue fever.Vaccine. 2011 Sep 23;29(42):7251-60. doi: 10.1016/j.vaccine.2011.07.020. Epub 2011 Jul 21. Vaccine. 2011. PMID: 21777638 Free PMC article. Review.
-
Dimerization of Dengue Virus E Subunits Impacts Antibody Function and Domain Focus.J Virol. 2020 Aug 31;94(18):e00745-20. doi: 10.1128/JVI.00745-20. Print 2020 Aug 31. J Virol. 2020. PMID: 32611757 Free PMC article.
-
Safety and Immunogenicity of a Live Attenuated Tetravalent Dengue Vaccine Candidate in Flavivirus-Naive Adults: A Randomized, Double-Blinded Phase 1 Clinical Trial.J Infect Dis. 2015 Oct 1;212(7):1032-41. doi: 10.1093/infdis/jiv179. Epub 2015 Mar 19. J Infect Dis. 2015. PMID: 25791116 Free PMC article. Clinical Trial.
-
Development of a recombinant, chimeric tetravalent dengue vaccine candidate.Vaccine. 2015 Dec 10;33(50):7112-20. doi: 10.1016/j.vaccine.2015.11.022. Epub 2015 Nov 14. Vaccine. 2015. PMID: 26585500 Review.
Cited by
-
A Review on Dengue Vaccine Development.Vaccines (Basel). 2020 Feb 2;8(1):63. doi: 10.3390/vaccines8010063. Vaccines (Basel). 2020. PMID: 32024238 Free PMC article. Review.
-
Development of Peptide Vaccines in Dengue.Curr Pharm Des. 2018;24(11):1157-1173. doi: 10.2174/1381612823666170913163904. Curr Pharm Des. 2018. PMID: 28914200 Free PMC article. Review.
-
Dengue vaccines: recent developments, ongoing challenges and current candidates.Expert Rev Vaccines. 2013 Aug;12(8):933-53. doi: 10.1586/14760584.2013.815412. Expert Rev Vaccines. 2013. PMID: 23984962 Free PMC article. Review.
-
Investigating the efficacy of monovalent and tetravalent dengue vaccine formulations against DENV-4 challenge in AG129 mice.Vaccine. 2014 Nov 12;32(48):6537-43. doi: 10.1016/j.vaccine.2014.08.087. Epub 2014 Sep 17. Vaccine. 2014. PMID: 25239488 Free PMC article.
-
Mouse models of dengue virus infection for vaccine testing.Vaccine. 2015 Dec 10;33(50):7051-60. doi: 10.1016/j.vaccine.2015.09.112. Epub 2015 Oct 23. Vaccine. 2015. PMID: 26478201 Free PMC article. Review.
References
-
- Mackenzie JS, Gubler DJ, Petersen LR. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nat Med. 2004;10:S98. - PubMed
-
- Wilder-Smith A, Gubler DJ. Geographic expansion of dengue: the impact of international travel. Med Clin North Am. 2008;92:1377–90. - PubMed
-
- Guzman MG, Kouri G. Dengue and dengue hemorrhagic fever in the Americas: lessons and challenges. J Clin Virol. 2003;27:1–13. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

