The methylthiolation reaction mediated by the Radical-SAM enzymes
- PMID: 22178611
- PMCID: PMC4150254
- DOI: 10.1016/j.bbapap.2011.11.007
The methylthiolation reaction mediated by the Radical-SAM enzymes
Abstract
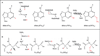
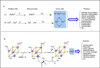
Over the past 10 years, considerable progress has been made in our understanding of the mechanistic enzymology of the Radical-SAM enzymes. It is now clear that these enzymes appear to be involved in a remarkably wide range of chemically challenging reactions. This review article highlights mechanistic and structural aspects of the methylthiotransferases (MTTases) sub-class of the Radical-SAM enzymes. The mechanism of methylthio insertion, now observed to be performed by three different enzymes is an exciting unsolved problem. This article is part of a Special Issue entitled: Radical SAM enzymes and Radical Enzymology.
Copyright © 2011 Elsevier B.V. All rights reserved.
Figures


Similar articles
-
Biotin synthase: insights into radical-mediated carbon-sulfur bond formation.Biochim Biophys Acta. 2012 Nov;1824(11):1213-22. doi: 10.1016/j.bbapap.2012.01.010. Epub 2012 Jan 28. Biochim Biophys Acta. 2012. PMID: 22326745 Review.
-
Recent advances in radical SAM enzymology: new structures and mechanisms.ACS Chem Biol. 2014 Sep 19;9(9):1929-38. doi: 10.1021/cb5004674. Epub 2014 Jul 16. ACS Chem Biol. 2014. PMID: 25009947 Free PMC article. Review.
-
Two Fe-S clusters catalyze sulfur insertion by radical-SAM methylthiotransferases.Nat Chem Biol. 2013 May;9(5):333-8. doi: 10.1038/nchembio.1229. Epub 2013 Mar 31. Nat Chem Biol. 2013. PMID: 23542644 Free PMC article.
-
Paramagnetic intermediates generated by radical S-adenosylmethionine (SAM) enzymes.Acc Chem Res. 2014 Aug 19;47(8):2235-43. doi: 10.1021/ar400235n. Epub 2014 Jul 3. Acc Chem Res. 2014. PMID: 24991701 Free PMC article.
-
Auxiliary iron-sulfur cofactors in radical SAM enzymes.Biochim Biophys Acta. 2015 Jun;1853(6):1316-34. doi: 10.1016/j.bbamcr.2015.01.002. Epub 2015 Jan 15. Biochim Biophys Acta. 2015. PMID: 25597998 Review.
Cited by
-
Physical and functional interactions of a monothiol glutaredoxin and an iron sulfur cluster carrier protein with the sulfur-donating radical S-adenosyl-L-methionine enzyme MiaB.J Biol Chem. 2013 May 17;288(20):14200-14211. doi: 10.1074/jbc.M113.460360. Epub 2013 Mar 29. J Biol Chem. 2013. PMID: 23543739 Free PMC article.
-
Methylated nucleosides in tRNA and tRNA methyltransferases.Front Genet. 2014 May 23;5:144. doi: 10.3389/fgene.2014.00144. eCollection 2014. Front Genet. 2014. PMID: 24904644 Free PMC article. Review.
-
Current Advancements in Sactipeptide Natural Products.Front Chem. 2021 May 20;9:595991. doi: 10.3389/fchem.2021.595991. eCollection 2021. Front Chem. 2021. PMID: 34095082 Free PMC article. Review.
-
Mobilization of Iron Stored in Bacterioferritin Is Required for Metabolic Homeostasis in Pseudomonas aeruginosa.Pathogens. 2020 Nov 24;9(12):980. doi: 10.3390/pathogens9120980. Pathogens. 2020. PMID: 33255203 Free PMC article.
-
First Step in Catalysis of the Radical S-Adenosylmethionine Methylthiotransferase MiaB Yields an Intermediate with a [3Fe-4S]0-Like Auxiliary Cluster.J Am Chem Soc. 2020 Jan 29;142(4):1911-1924. doi: 10.1021/jacs.9b11093. Epub 2020 Jan 16. J Am Chem Soc. 2020. PMID: 31899624 Free PMC article.
References
-
- Eisenstein O, Balcells D, Clot E. C-H Bond Activation in Transition Metal Species from a Computational Perspective. Chem. Rev. 2010;110:749–823. - PubMed
-
- Que L, Costas M, Mehn MP, Jensen MP. Dioxygen activation at mononuclear nonheme iron active sites: Enzymes, models, and intermediates. Chem. Rev. 2004;104:939–986. - PubMed
-
- Lippard SJ. Hydroxylation of C-H bonds at carboxylate-bridged diiron centres. Philosophical Transactions of the Royal Society a-Mathematical Physical and Engineering Sciences. 2005;363:861–877. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

