Viral interferon regulatory factors are critical for delay of the host immune response against rhesus macaque rhadinovirus infection
- PMID: 22171275
- PMCID: PMC3302252
- DOI: 10.1128/JVI.05657-11
Viral interferon regulatory factors are critical for delay of the host immune response against rhesus macaque rhadinovirus infection
Abstract
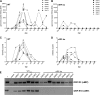
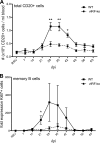
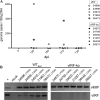
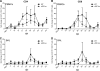
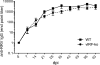
Kaposi's sarcoma-associated herpesvirus (KSHV) and the closely related gamma-2 herpesvirus rhesus macaque (RM) rhadinovirus (RRV) are the only known viruses to encode viral homologues of the cellular interferon (IFN) regulatory factors (IRFs). Recent characterization of a viral IRF (vIRF) deletion clone of RRV (vIRF-knockout RRV [vIRF-ko RRV]) demonstrated that vIRFs inhibit induction of type I and type II IFNs during RRV infection of peripheral blood mononuclear cells. Because the IFN response is a key component to a host's antiviral defenses, this study has investigated the role of vIRFs in viral replication and the development of the immune response during in vivo infection in RMs, the natural host of RRV. Experimental infection of RMs with vIRF-ko RRV resulted in decreased viral loads and diminished B cell hyperplasia, a characteristic pathology during acute RRV infection that often develops into more severe lymphoproliferative disorders in immune-compromised animals, similar to pathologies in KSHV-infected individuals. Moreover, in vivo infection with vIRF-ko RRV resulted in earlier and sustained production of proinflammatory cytokines and earlier induction of an anti-RRV T cell response compared to wild-type RRV infection. These findings reveal the broad impact that vIRFs have on pathogenesis and the immune response in vivo and are the first to validate the importance of vIRFs during de novo infection in the host.
Figures


Similar articles
-
A Rhesus Rhadinovirus Viral Interferon (IFN) Regulatory Factor Is Virion Associated and Inhibits the Early IFN Antiviral Response.J Virol. 2015 Aug;89(15):7707-21. doi: 10.1128/JVI.01175-15. Epub 2015 May 13. J Virol. 2015. PMID: 25972548 Free PMC article.
-
Viral interferon regulatory factors decrease the induction of type I and type II interferon during rhesus macaque rhadinovirus infection.J Virol. 2012 Feb;86(4):2197-211. doi: 10.1128/JVI.05047-11. Epub 2011 Dec 7. J Virol. 2012. PMID: 22156526 Free PMC article.
-
Rhesus Macaque Rhadinovirus Encodes a Viral Interferon Regulatory Factor To Disrupt Promyelocytic Leukemia Nuclear Bodies and Antagonize Type I Interferon Signaling.J Virol. 2019 Mar 5;93(6):e02147-18. doi: 10.1128/JVI.02147-18. Print 2019 Mar 15. J Virol. 2019. PMID: 30626678 Free PMC article.
-
Rhesus macaque rhadinovirus-associated disease.Curr Opin Virol. 2013 Jun;3(3):245-50. doi: 10.1016/j.coviro.2013.05.016. Epub 2013 Jun 6. Curr Opin Virol. 2013. PMID: 23747119 Free PMC article. Review.
-
Viral interferon regulatory factors.J Interferon Cytokine Res. 2009 Sep;29(9):621-7. doi: 10.1089/jir.2009.0067. J Interferon Cytokine Res. 2009. PMID: 19715458 Free PMC article. Review.
Cited by
-
B Cell-Intrinsic Expression of Interferon Regulatory Factor 1 Supports Chronic Murine Gammaherpesvirus 68 Infection.J Virol. 2020 Jun 16;94(13):e00399-20. doi: 10.1128/JVI.00399-20. Print 2020 Jun 16. J Virol. 2020. PMID: 32321819 Free PMC article.
-
Biologically significant interaction of human herpesvirus 8 viral interferon regulatory factor 4 with ubiquitin-specific protease 7.J Virol. 2024 Jun 13;98(6):e0025524. doi: 10.1128/jvi.00255-24. Epub 2024 May 16. J Virol. 2024. PMID: 38752725 Free PMC article.
-
Distinct roles of Kaposi's sarcoma-associated herpesvirus-encoded viral interferon regulatory factors in inflammatory response and cancer.J Virol. 2013 Sep;87(17):9398-410. doi: 10.1128/JVI.03315-12. Epub 2013 Jun 19. J Virol. 2013. PMID: 23785197 Free PMC article. Review.
-
A Rhesus Rhadinovirus Viral Interferon (IFN) Regulatory Factor Is Virion Associated and Inhibits the Early IFN Antiviral Response.J Virol. 2015 Aug;89(15):7707-21. doi: 10.1128/JVI.01175-15. Epub 2015 May 13. J Virol. 2015. PMID: 25972548 Free PMC article.
-
The viral interferon regulatory factors of kaposi's sarcoma-associated herpesvirus differ in their inhibition of interferon activation mediated by toll-like receptor 3.J Virol. 2013 Jan;87(2):798-806. doi: 10.1128/JVI.01851-12. Epub 2012 Oct 31. J Virol. 2013. PMID: 23115281 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

