Measles virus causes immunogenic cell death in human melanoma
- PMID: 22170342
- PMCID: PMC3378495
- DOI: 10.1038/gt.2011.205
Measles virus causes immunogenic cell death in human melanoma
Abstract
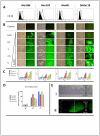
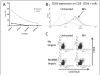
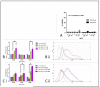
Oncolytic viruses (OV) are promising treatments for cancer, with several currently undergoing testing in randomised clinical trials. Measles virus (MV) has not yet been tested in models of human melanoma. This study demonstrates the efficacy of MV against human melanoma. It is increasingly recognised that an essential component of therapy with OV is the recruitment of host antitumour immune responses, both innate and adaptive. MV-mediated melanoma cell death is an inflammatory process, causing the release of inflammatory cytokines including type-1 interferons and the potent danger signal HMGB1. Here, using human in vitro models, we demonstrate that MV enhances innate antitumour activity, and that MV-mediated melanoma cell death is capable of stimulating a melanoma-specific adaptive immune response.
Figures



Similar articles
-
The Susceptibility of Human Melanoma Cells to Infection with the Leningrad-16 Vaccine Strain of Measles Virus.Viruses. 2020 Feb 4;12(2):173. doi: 10.3390/v12020173. Viruses. 2020. PMID: 32033013 Free PMC article.
-
Modulation of the Type I Interferon Response Defines the Sensitivity of Human Melanoma Cells to Oncolytic Measles Virus.Curr Gene Ther. 2017;16(6):419-428. doi: 10.2174/1566523217666170102110502. Curr Gene Ther. 2017. PMID: 28042780
-
Systemically delivered measles virus-infected mesenchymal stem cells can evade host immunity to inhibit liver cancer growth.J Hepatol. 2013 Nov;59(5):999-1006. doi: 10.1016/j.jhep.2013.07.010. Epub 2013 Jul 16. J Hepatol. 2013. PMID: 23867315 Free PMC article.
-
Therapeutic potential of oncolytic measles virus: promises and challenges.Clin Pharmacol Ther. 2010 Nov;88(5):620-5. doi: 10.1038/clpt.2010.211. Epub 2010 Sep 29. Clin Pharmacol Ther. 2010. PMID: 20881957 Review.
-
Measles virus for cancer therapy.Curr Top Microbiol Immunol. 2009;330:213-41. doi: 10.1007/978-3-540-70617-5_11. Curr Top Microbiol Immunol. 2009. PMID: 19203112 Free PMC article. Review.
Cited by
-
Oncolytic viruses as immunotherapy: progress and remaining challenges.Onco Targets Ther. 2016 May 4;9:2627-37. doi: 10.2147/OTT.S63049. eCollection 2016. Onco Targets Ther. 2016. PMID: 27226725 Free PMC article. Review.
-
Metabolic Reprogramming by Reduced Calorie Intake or Pharmacological Caloric Restriction Mimetics for Improved Cancer Immunotherapy.Cancers (Basel). 2021 Mar 12;13(6):1260. doi: 10.3390/cancers13061260. Cancers (Basel). 2021. PMID: 33809187 Free PMC article. Review.
-
The MCIB Model: A Novel Theory for Describing the Spatial Heterogeneity of the Tumor Microenvironment.Int J Mol Sci. 2024 Sep 29;25(19):10486. doi: 10.3390/ijms251910486. Int J Mol Sci. 2024. PMID: 39408814 Free PMC article. Review.
-
Recent Advances and Next Breakthrough in Immunotherapy for Cancer Treatment.J Immunol Res. 2022 Mar 18;2022:8052212. doi: 10.1155/2022/8052212. eCollection 2022. J Immunol Res. 2022. PMID: 35340585 Free PMC article. Review.
-
The Susceptibility of Human Melanoma Cells to Infection with the Leningrad-16 Vaccine Strain of Measles Virus.Viruses. 2020 Feb 4;12(2):173. doi: 10.3390/v12020173. Viruses. 2020. PMID: 32033013 Free PMC article.
References
-
- Grote D, et al. Live attenuated measles virus induces regression of human lymphoma xenografts in immunodeficient mice. Blood. 2001;97:3746–3754. - PubMed
-
- Peng KW, et al. Systemic therapy of myeloma xenografts by an attenuated measles virus. Blood. 2001;98:2002–7. - PubMed
-
- Peng K-W, et al. Intraperitoneal Therapy of Ovarian Cancer Using an Engineered Measles Virus. Cancer Res. 2002;62:4656–4662. - PubMed
-
- Phuong LK, et al. Use of a Vaccine Strain of Measles Virus Genetically Engineered to Produce Carcinoembryonic Antigen as a Novel Therapeutic Agent against Glioblastoma Multiforme. Cancer Research. 2003;63:2462–2469. - PubMed
-
- Peng K-W, et al. Biodistribution of oncolytic measles virus after intraperitoneal administration into Ifnar-CD46Ge transgenic mice. Hum. Gene Ther. 2003;14:1565–1577. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

