Subcellular targeting domains of sphingomyelin synthase 1 and 2
- PMID: 22168400
- PMCID: PMC3264500
- DOI: 10.1186/1743-7075-8-89
Subcellular targeting domains of sphingomyelin synthase 1 and 2
Abstract
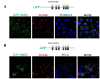
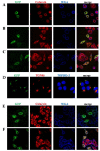
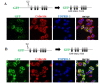
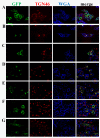
Sphingomyelin synthase (SMS) sits at the crossroads of sphingomyelin (SM), ceramide, diacylglycerol (DAG) metabolism. It utilizes ceramide and phosphatidylcholine as substrates to produce SM and DAG, thereby regulating lipid messengers which play a role in cell survival and apoptosis. Furthermore, its product SM has been implicated in atherogenic processes such as retention of lipoproteins in the blood vessel intima. There are two mammalian sphingomyelin synthases: SMS1 and SMS2. SMS1 is found exclusively in the Golgi at steady state, whereas SMS2 exists in the Golgi and plasma membrane. Conventional motifs responsible for protein targeting to the plasma membrane or Golgi are either not present in, or unique to, SMS1 and SMS2. In this study, we examined how SMS1 and SMS2 achieve their respective subcellular localization patterns. Brefeldin A treatment prevented SMS1 and SMS2 from exiting the ER, demonstrating that they transit through the classical secretory pathway. We created truncations and chimeras of SMS1 and SMS2 to define their targeting signals. We found that SMS1 contains a C-terminal Golgi targeting signal and that SMS2 contains a C-terminal plasma membrane targeting signal.
Figures

Similar articles
-
Inhibition of sphingomyelin synthase (SMS) affects intracellular sphingomyelin accumulation and plasma membrane lipid organization.Biochim Biophys Acta. 2007 Sep;1771(9):1186-94. doi: 10.1016/j.bbalip.2007.05.007. Epub 2007 Jun 6. Biochim Biophys Acta. 2007. PMID: 17616479 Free PMC article.
-
SMS overexpression and knockdown: impact on cellular sphingomyelin and diacylglycerol metabolism, and cell apoptosis.J Lipid Res. 2008 Feb;49(2):376-85. doi: 10.1194/jlr.M700401-JLR200. Epub 2007 Nov 2. J Lipid Res. 2008. PMID: 17982138
-
Sphingomyelin synthases regulate production of diacylglycerol at the Golgi.Biochem J. 2008 Aug 15;414(1):31-41. doi: 10.1042/BJ20071240. Biochem J. 2008. PMID: 18370930 Free PMC article.
-
The sphingomyelin synthase family: proteins, diseases, and inhibitors.Biol Chem. 2017 Nov 27;398(12):1319-1325. doi: 10.1515/hsz-2017-0148. Biol Chem. 2017. PMID: 28742512 Review.
-
Role of ceramide/sphingomyelin (SM) balance regulated through "SM cycle" in cancer.Cell Signal. 2021 Nov;87:110119. doi: 10.1016/j.cellsig.2021.110119. Epub 2021 Aug 19. Cell Signal. 2021. PMID: 34418535 Review.
Cited by
-
Sphingomyelin synthase 2 overexpression promotes cisplatin-induced apoptosis of HepG2 cells.Oncol Lett. 2018 Jan;15(1):483-488. doi: 10.3892/ol.2017.7309. Epub 2017 Oct 31. Oncol Lett. 2018. PMID: 29375716 Free PMC article.
-
Sphingomyelin synthase-related protein generates diacylglycerol via the hydrolysis of glycerophospholipids in the absence of ceramide.J Biol Chem. 2021 Jan-Jun;296:100454. doi: 10.1016/j.jbc.2021.100454. Epub 2021 Feb 20. J Biol Chem. 2021. PMID: 33621517 Free PMC article.
-
Sphingomyelin Synthase 2 Participate in the Regulation of Sperm Motility and Apoptosis.Molecules. 2020 Sep 15;25(18):4231. doi: 10.3390/molecules25184231. Molecules. 2020. PMID: 32942681 Free PMC article.
-
Ceramide/Sphingomyelin Rheostat Regulated by Sphingomyelin Synthases and Chronic Diseases in Murine Models.J Lipid Atheroscler. 2020 Sep;9(3):380-405. doi: 10.12997/jla.2020.9.3.380. Epub 2020 Jul 29. J Lipid Atheroscler. 2020. PMID: 33024732 Free PMC article. Review.
-
Carboxyl-terminal Tail-mediated Homodimerizations of Sphingomyelin Synthases Are Responsible for Efficient Export from the Endoplasmic Reticulum.J Biol Chem. 2017 Jan 20;292(3):1122-1141. doi: 10.1074/jbc.M116.746602. Epub 2016 Dec 7. J Biol Chem. 2017. PMID: 27927984 Free PMC article.
References
LinkOut - more resources
Full Text Sources

