A C-terminal tyrosine-based motif in the bile salt export pump directs clathrin-dependent endocytosis
- PMID: 22161577
- PMCID: PMC3319652
- DOI: 10.1002/hep.25523
A C-terminal tyrosine-based motif in the bile salt export pump directs clathrin-dependent endocytosis
Abstract
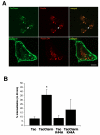
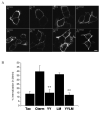
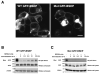
The liver-specific bile salt export pump (BSEP) is crucial for bile acid-dependent bile flow at the apical membrane. BSEP, a member of the family of structurally related adenosine triphosphate (ATP)-binding cassette (ABC) proteins, is composed of 12 transmembrane segments (TMS) and two large cytoplasmic nucleotide-binding domains (NBDs). The regulation of trafficking of BSEP to and from the cell surface is not well understood, but is believed to play an important role in cholestatic liver diseases such as primary familial intrahepatic cholestasis type 2 (PFIC2). To address this issue, BSEP endocytosis was studied by immunofluorescence and a cell surface enzyme-linked immunosorbent assay (ELISA) endocytosis reporter system using a chimera of the interleukin-2 receptor α (previously referred to as Tac) and the C-terminal tail of BSEP (TacCterm). An autonomous endocytosis motif in the carboxyl cytoplasmic terminus of BSEP was identified. We define this endocytic motif by site-directed mutagenesis as a canonical tyrosine-based motif (1310) YYKLV(1314) (YxxØ). When expressed in HEK293T cells, TacCterm is constitutively internalized via a dynamin- and clathrin-dependent pathway. Mutation of the Y(1310) Y(1311) amino acids in TacCterm and in full-length human BSEP blocks the internalization. Subsequent sequence analysis reveals this motif to be highly conserved between the closely related ABCB subfamily members that mediate ATP-dependent transport of broad substrate specificity.
Conclusion: Our results indicate that constitutive internalization of BSEP is clathrin-mediated and dependent on the tyrosine-based endocytic motif at the C-terminal end of BSEP.
Copyright © 2012 American Association for the Study of Liver Diseases.
Figures



Similar articles
-
AP2 adaptor complex mediates bile salt export pump internalization and modulates its hepatocanalicular expression and transport function.Hepatology. 2012 Jun;55(6):1889-900. doi: 10.1002/hep.25591. Hepatology. 2012. PMID: 22262466
-
Differential roles of ubiquitination in the degradation mechanism of cell surface-resident bile salt export pump and multidrug resistance-associated protein 2.Mol Pharmacol. 2014 Mar;85(3):482-91. doi: 10.1124/mol.113.091090. Epub 2013 Dec 30. Mol Pharmacol. 2014. PMID: 24378332
-
Identification of HAX-1 as a protein that binds bile salt export protein and regulates its abundance in the apical membrane of Madin-Darby canine kidney cells.J Biol Chem. 2004 Jul 30;279(31):32761-70. doi: 10.1074/jbc.M404337200. Epub 2004 May 24. J Biol Chem. 2004. PMID: 15159385
-
Bile salt excretory pump: biology and pathobiology.J Pediatr Gastroenterol Nutr. 2006 Jul;43 Suppl 1:S10-6. doi: 10.1097/01.mpg.0000226385.71859.5f. J Pediatr Gastroenterol Nutr. 2006. PMID: 16819395 Review.
-
The bile salt export pump: clinical and experimental aspects of genetic and acquired cholestatic liver disease.Semin Liver Dis. 2010 May;30(2):125-33. doi: 10.1055/s-0030-1253222. Epub 2010 Apr 26. Semin Liver Dis. 2010. PMID: 20422495 Free PMC article. Review.
Cited by
-
Bile formation and secretion.Compr Physiol. 2013 Jul;3(3):1035-78. doi: 10.1002/cphy.c120027. Compr Physiol. 2013. PMID: 23897680 Free PMC article. Review.
-
ERK1/2 and p38 MAPKs are complementarily involved in estradiol 17ß-D-glucuronide-induced cholestasis: crosstalk with cPKC and PI3K.PLoS One. 2012;7(11):e49255. doi: 10.1371/journal.pone.0049255. Epub 2012 Nov 14. PLoS One. 2012. PMID: 23166621 Free PMC article.
-
Recombinant tandem of pore-domains in a Weakly Inward rectifying K+ channel 2 (TWIK2) forms active lysosomal channels.Sci Rep. 2017 Apr 5;7(1):649. doi: 10.1038/s41598-017-00640-8. Sci Rep. 2017. PMID: 28381826 Free PMC article.
-
Systematic Assessment of Protein C-Termini Mutated in Human Disorders.Biomolecules. 2023 Feb 12;13(2):355. doi: 10.3390/biom13020355. Biomolecules. 2023. PMID: 36830724 Free PMC article.
-
MRCK-Alpha and Its Effector Myosin II Regulatory Light Chain Bind ABCB4 and Regulate Its Membrane Expression.Cells. 2022 Feb 10;11(4):617. doi: 10.3390/cells11040617. Cells. 2022. PMID: 35203270 Free PMC article.
References
-
- Jansen PL, Strautnieks SS, Jacquemin E, Hadchouel M, Sokal EM, Hooiveld GJ, Koning JH, et al. Hepatocanalicular bile salt export pump deficiency in patients with progressive familial intrahepatic cholestasis. Gastroenterology. 1999;117:1370–1379. - PubMed
-
- Strautnieks SS, Bull LN, Knisely AS, Kocoshis SA, Dahl N, Arnell H, Sokal E, et al. A gene encoding a liver-specific ABC transporter is mutated in progressive familial intrahepatic cholestasis. Nat Genet. 1998;20:233–238. - PubMed
-
- Strautnieks SS, Byrne JA, Pawlikowska L, Cebecauerova D, Rayner A, Dutton L, Meier Y, et al. Severe bile salt export pump deficiency: 82 different ABCB11 mutations in 109 families. Gastroenterology. 2008;134:1203–1214. - PubMed
-
- Kipp H, Pichetshote N, Arias IM. Transporters on demand: intrahepatic pools of canalicular ATP binding cassette transporters in rat liver. Journal of Biological Chemistry. 2001;276:7218–7224. - PubMed
-
- Kipp H, Arias IM. Newly synthesized canalicular ABC transporters are directly targeted from the Golgi to the hepatocyte apical domain in rat liver. Journal of Biological Chemistry. 2000;275:15917–15925. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
