Prostacyclin receptor-mediated ATP release from erythrocytes requires the voltage-dependent anion channel
- PMID: 22159995
- PMCID: PMC3353798
- DOI: 10.1152/ajpheart.00998.2011
Prostacyclin receptor-mediated ATP release from erythrocytes requires the voltage-dependent anion channel
Abstract
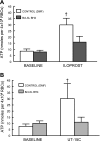
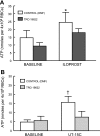
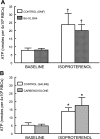
Erythrocytes have been implicated as controllers of vascular caliber by virtue of their ability to release the vasodilator ATP in response to local physiological and pharmacological stimuli. The regulated release of ATP from erythrocytes requires activation of a signaling pathway involving G proteins (G(i) or G(s)), adenylyl cyclase, protein kinase A, and the cystic fibrosis transmembrane conductance regulator as well as a final conduit through which this highly charged anion exits the cell. Although pannexin 1 has been shown to be the final conduit for ATP release from human erythrocytes in response to reduced oxygen tension, it does not participate in transport of ATP following stimulation of the prostacyclin (IP) receptor in these cells, which suggests that an additional protein must be involved. Using antibodies directed against voltage-dependent anion channel (VDAC)1, we confirm that this protein is present in human erythrocyte membranes. To address the role of VDAC in ATP release, two structurally dissimilar VDAC inhibitors, Bcl-x(L) BH4(4-23) and TRO19622, were used. In response to the IP receptor agonists, iloprost and UT-15C, ATP release was inhibited by both VDAC inhibitors although neither iloprost-induced cAMP accumulation nor total intracellular ATP concentration were altered. Together, these findings support the hypothesis that VDAC is the ATP conduit in the IP receptor-mediated signaling pathway in human erythrocytes. In addition, neither the pannexin inhibitor carbenoxolone nor Bcl-x(L) BH4(4-23) attenuated ATP release in response to incubation of erythrocytes with the β-adrenergic receptor agonist isoproterenol, suggesting the presence of yet another channel for ATP release from human erythrocytes.
Figures

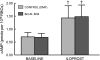
Similar articles
-
Pannexin 1 is the conduit for low oxygen tension-induced ATP release from human erythrocytes.Am J Physiol Heart Circ Physiol. 2010 Oct;299(4):H1146-52. doi: 10.1152/ajpheart.00301.2010. Epub 2010 Jul 9. Am J Physiol Heart Circ Physiol. 2010. PMID: 20622111 Free PMC article.
-
Prostacyclin analogs stimulate receptor-mediated cAMP synthesis and ATP release from rabbit and human erythrocytes.Microcirculation. 2008 Jul;15(5):461-71. doi: 10.1080/10739680701833804. Microcirculation. 2008. PMID: 18574748 Free PMC article.
-
Iloprost- and isoproterenol-induced increases in cAMP are regulated by different phosphodiesterases in erythrocytes of both rabbits and humans.Am J Physiol Heart Circ Physiol. 2009 May;296(5):H1617-24. doi: 10.1152/ajpheart.01226.2008. Epub 2009 Feb 27. Am J Physiol Heart Circ Physiol. 2009. PMID: 19252089 Free PMC article.
-
New insights into the intracellular mechanisms by which PGI2 analogues elicit vascular relaxation: cyclic AMP-independent, Gs-protein mediated-activation of MaxiK channel.Curr Med Chem Cardiovasc Hematol Agents. 2004 Jul;2(3):257-65. doi: 10.2174/1568016043356273. Curr Med Chem Cardiovasc Hematol Agents. 2004. PMID: 15320791 Review.
-
Regulation of cAMP by phosphodiesterases in erythrocytes.Pharmacol Rep. 2010 May-Jun;62(3):475-82. doi: 10.1016/s1734-1140(10)70303-0. Pharmacol Rep. 2010. PMID: 20631411 Free PMC article. Review.
Cited by
-
Effects of Hypoxia on Erythrocyte Membrane Properties-Implications for Intravascular Hemolysis and Purinergic Control of Blood Flow.Front Physiol. 2017 Dec 22;8:1110. doi: 10.3389/fphys.2017.01110. eCollection 2017. Front Physiol. 2017. PMID: 29312010 Free PMC article. Review.
-
Role of erythrocyte-released ATP in the regulation of microvascular oxygen supply in skeletal muscle.Acta Physiol (Oxf). 2016 Mar;216(3):265-76. doi: 10.1111/apha.12596. Epub 2015 Sep 25. Acta Physiol (Oxf). 2016. PMID: 26336065 Free PMC article. Review.
-
Purinergic control of red blood cell metabolism: novel strategies to improve red cell storage quality.Blood Transfus. 2017 Oct;15(6):535-542. doi: 10.2450/2017.0366-16. Epub 2017 Apr 12. Blood Transfus. 2017. PMID: 28488967 Free PMC article. Review.
-
The pannexins: past and present.Front Physiol. 2014 Feb 19;5:58. doi: 10.3389/fphys.2014.00058. eCollection 2014. Front Physiol. 2014. PMID: 24600404 Free PMC article. Review.
-
Hemolysis is a primary ATP-release mechanism in human erythrocytes.Blood. 2014 Sep 25;124(13):2150-7. doi: 10.1182/blood-2014-05-572024. Epub 2014 Aug 5. Blood. 2014. PMID: 25097178 Free PMC article.
References
-
- Abraham EH, Okunieff P, Scala S, Vos P, Oosterveld MJ, Chen AY, Shrivastav B. Cystic fibrosis transmembrane conductance regulator and adenosine triphosphate. Science 275: 1324–1326, 1997 - PubMed
-
- Abraham EH, Sterling KM, Kim RJ, Salikhova AY, Huffman HB, Crockett MA, Johnston N, Parker HW, Boyle WE, Jr, Hartov A, Demidenko E, Efird J, Kahn J, Grubman SA, Jefferson DM, Robson SC, Thakar JH, Lorico A, Rappa G, Sartorelli AC, Okunieff P. Erythrocyte membrane ATP binding cassette (ABC) proteins: MRP1 and CFTR as well as CD39 (ecto-apyrase) involved in RBC ATP transport and elevated blood plasma ATP of cystic fibrosis. Blood Cells Mol Dis 27: 165–180, 2001 - PubMed
-
- Adderley SP, Dufaux EA, Sridharan M, Bowles EA, Hanson MS, Stephenson AH, Ellsworth ML, Sprague RS. Iloprost- and isoproterenol-induced increases in cAMP are regulated by different phosphodiesterases in erythrocytes of both rabbits and humans. Am J Physiol Heart Circ Physiol 296: H1617–H1624, 2009 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

