Neuron-specific effects of interleukin-1β are mediated by a novel isoform of the IL-1 receptor accessory protein
- PMID: 22159118
- PMCID: PMC3261076
- DOI: 10.1523/JNEUROSCI.4067-11.2011
Neuron-specific effects of interleukin-1β are mediated by a novel isoform of the IL-1 receptor accessory protein
Abstract
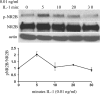
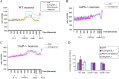
In the CNS, interleukin-1β (IL-1β) is synthesized and released during injury, infection, and disease, mediating inflammatory responses. However, IL-1β is also present in the brain under physiological conditions, and can influence hippocampal neuronal function. Several cell-specific IL-1-mediated signaling pathways and functions have been identified in neurons and astrocytes, but their mechanisms have not been fully defined. In astrocytes, IL-1β induced both the p38 MAPK and NF-κB (nuclear factor κB) pathways regulating inflammatory responses, however in hippocampal neurons IL-1β activated p38 but not NF-κB. Additionally, IL-1β induced Src phosphorylation at 0.01 ng/ml in hippocampal neurons, a dose 1000-fold lower than that used to stimulate inflammatory responses. IL-1 signaling requires the type 1 IL-1 receptor and the IL-1 receptor accessory protein (IL-1RAcP) as a receptor partner. We previously reported a novel isoform of the IL-1RAcP, IL-1RAcPb, found exclusively in CNS neurons. In this study, we demonstrate that AcPb specifically mediates IL-1β activation of p-Src and potentiation of NMDA-induced calcium influx in mouse hippocampal neurons in a dose-dependent manner. Mice lacking the AcPb, but retaining the AcP, isoform were deficient in IL-1β regulation of p-Src in neurons. AcPb also played a modulatory role in the activation of p38 MAPK, but had no effect on NF-κB signaling. The restricted expression of AcPb in CNS neurons, therefore, governs specific neuronal signaling and functional responses to IL-1β.
Figures

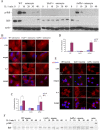
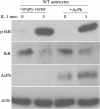
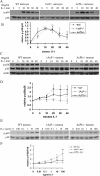

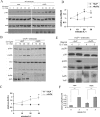
Similar articles
-
Cell type-specific interleukin-1beta signaling in the CNS.J Neurosci. 2004 Jul 21;24(29):6482-8. doi: 10.1523/JNEUROSCI.5712-03.2004. J Neurosci. 2004. PMID: 15269258 Free PMC article.
-
Contribution of interleukin-1 receptor accessory protein B to interleukin-1 actions in neuronal cells.Neurosignals. 2011;19(4):222-30. doi: 10.1159/000330803. Epub 2011 Sep 28. Neurosignals. 2011. PMID: 21968452
-
Acute p38-mediated inhibition of NMDA-induced outward currents in hippocampal CA1 neurons by interleukin-1beta.Neurobiol Dis. 2010 Apr;38(1):68-77. doi: 10.1016/j.nbd.2009.12.028. Epub 2010 Jan 11. Neurobiol Dis. 2010. PMID: 20060906
-
Synapse-specific IL-1 receptor subunit reconfiguration augments vulnerability to IL-1β in the aged hippocampus.Proc Natl Acad Sci U S A. 2015 Sep 8;112(36):E5078-87. doi: 10.1073/pnas.1514486112. Epub 2015 Aug 24. Proc Natl Acad Sci U S A. 2015. PMID: 26305968 Free PMC article.
-
The roles of interleukin-1 receptor accessory protein in certain inflammatory conditions.Immunology. 2022 May;166(1):38-46. doi: 10.1111/imm.13462. Epub 2022 Mar 10. Immunology. 2022. PMID: 35231129 Review.
Cited by
-
Molecular crosstalk between circadian clock and NLRP3 inflammasome signaling in Parkinson's disease.Heliyon. 2024 Jan 14;10(2):e24752. doi: 10.1016/j.heliyon.2024.e24752. eCollection 2024 Jan 30. Heliyon. 2024. PMID: 38268831 Free PMC article. Review.
-
Anti-HMGB1 auto-Abs influence fatigue in patients with Crohn's disease.Innate Immun. 2021 May;27(4):286-293. doi: 10.1177/17534259211014252. Epub 2021 May 3. Innate Immun. 2021. PMID: 33940970 Free PMC article.
-
Src Family Kinases Facilitate the Crosstalk between CGRP and Cytokines in Sensitizing Trigeminal Ganglion via Transmitting CGRP Receptor/PKA Pathway.Cells. 2022 Nov 4;11(21):3498. doi: 10.3390/cells11213498. Cells. 2022. PMID: 36359895 Free PMC article.
-
Sleep- and time of day-linked RNA transcript expression in wild-type and IL1 receptor accessory protein-null mice.J Appl Physiol (1985). 2020 Jun 1;128(6):1506-1522. doi: 10.1152/japplphysiol.00839.2019. Epub 2020 Apr 23. J Appl Physiol (1985). 2020. PMID: 32324480 Free PMC article.
-
The role of brain interleukin-1 in stress-enhanced fear learning.Neuropsychopharmacology. 2015 Mar 13;40(5):1289-96. doi: 10.1038/npp.2014.317. Neuropsychopharmacology. 2015. PMID: 25430780 Free PMC article.
References
-
- Andre R, Lerouet D, Kimber I, Pinteaux E, Rothwell NJ. Regulation of expression of the novel IL-1 receptor family members in the mouse brain. J Neurochem. 2005;95:324–330. - PubMed
-
- Avital A, Goshen I, Kamsler A, Segal M, Iverfeldt K, Richter-Levin G, Yirmiya R. Impaired interleukin-1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus. 2003;13:826–834. - PubMed
-
- Bellinger FP, Madamba S, Siggins GR. Interleukin 1β inhibits synaptic strength and long-term potentiation in the rat CA1 hippocampus. Brain Res. 1993;628:227–234. - PubMed
-
- Berglöf E, Andre R, Renshaw BR, Allan SM, Lawrence CB, Rothwell NJ, Pinteaux E. IL-1Rrp2 expression and IL-1F9 (IL-1H1) actions in brain cells. J Neuroimmunol. 2003;139:36–43. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
