Regenerating islet-derived 1α (Reg-1α) protein is new neuronal secreted factor that stimulates neurite outgrowth via exostosin Tumor-like 3 (EXTL3) receptor
- PMID: 22158612
- PMCID: PMC3281625
- DOI: 10.1074/jbc.M111.260349
Regenerating islet-derived 1α (Reg-1α) protein is new neuronal secreted factor that stimulates neurite outgrowth via exostosin Tumor-like 3 (EXTL3) receptor
Abstract
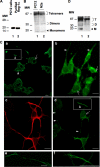
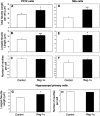
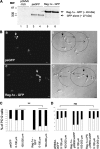
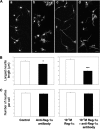
Regenerating islet-derived 1α (Reg-1α)/lithostathine, a member of a family of secreted proteins containing a C-type lectin domain, is expressed in various organs and plays a role in proliferation, differentiation, inflammation, and carcinogenesis of cells of the digestive system. We previously reported that Reg-1α is overexpressed during the very early stages of Alzheimer disease, and Reg-1α deposits were detected in the brain of patients with Alzheimer disease. However, the physiological function of Reg-1α in neural cells remains unknown. Here, we show that Reg-1α is expressed in neuronal cell lines (PC12 and Neuro-2a) and in rat primary hippocampal neurons (E17.5). Reg-1α is mainly localized around the nucleus and at the membrane of cell bodies and neurites. Transient overexpression of Reg-1α or addition of recombinant Reg-1α significantly increases the number of cells with longer neurites by stimulating neurite outgrowth. These effects are abolished upon down-regulation of Reg-1α by siRNA and following inhibition of secreted Reg-1α by antibodies. Moreover, Reg-1α colocalizes with exostosin tumor-like 3 (EXTL3), its putative receptor, at the membrane of these cells. Overexpression of EXTL3 increases the effect of recombinant Reg-1α on neurite outgrowth, and Reg-1α is not effective when EXTL3 overexpression is down-regulated by shRNA. Our findings indicate that Reg-1α regulates neurite outgrowth and suggest that this effect is mediated by its receptor EXTL3.
Figures



Similar articles
-
Specific functions of Exostosin-like 3 (EXTL3) gene products.Cell Mol Biol Lett. 2020 Aug 20;25:39. doi: 10.1186/s11658-020-00231-y. eCollection 2020. Cell Mol Biol Lett. 2020. PMID: 32843889 Free PMC article. Review.
-
Regenerating islet-derived 1α (REG-1α) protein increases tau phosphorylation in cell and animal models of tauopathies.Neurobiol Dis. 2018 Nov;119:136-148. doi: 10.1016/j.nbd.2018.07.029. Epub 2018 Aug 6. Neurobiol Dis. 2018. PMID: 30092268
-
Reg-1α Promotes Differentiation of Cortical Progenitors via Its N-Terminal Active Domain.Front Cell Dev Biol. 2020 Aug 13;8:681. doi: 10.3389/fcell.2020.00681. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 32903776 Free PMC article.
-
Regenerating islet-derived protein 1 inhibits the activation of islet stellate cells isolated from diabetic mice.Oncotarget. 2015 Nov 10;6(35):37054-65. doi: 10.18632/oncotarget.6163. Oncotarget. 2015. PMID: 26496027 Free PMC article.
-
The Reg gene family and Reg proteins: with special attention to the regeneration of pancreatic beta-cells.J Hepatobiliary Pancreat Surg. 1999;6(3):254-62. doi: 10.1007/s005340050115. J Hepatobiliary Pancreat Surg. 1999. PMID: 10526060 Review.
Cited by
-
Circulating Factors as Potential Biomarkers of Cardiovascular Damage Progression Associated with Type 2 Diabetes.Proteomes. 2024 Oct 11;12(4):29. doi: 10.3390/proteomes12040029. Proteomes. 2024. PMID: 39449501 Free PMC article.
-
Nerve Injury-Induced Neuronal PAP-I Maintains Neuropathic Pain by Activating Spinal Microglia.J Neurosci. 2020 Jan 8;40(2):297-310. doi: 10.1523/JNEUROSCI.1414-19.2019. Epub 2019 Nov 19. J Neurosci. 2020. PMID: 31744864 Free PMC article.
-
Altered gene expression in early postnatal monoamine oxidase A knockout mice.Brain Res. 2017 Aug 15;1669:18-26. doi: 10.1016/j.brainres.2017.05.017. Epub 2017 May 20. Brain Res. 2017. PMID: 28535982 Free PMC article.
-
Novel siRNA delivery strategy: a new "strand" in CNS translational medicine?Cell Mol Life Sci. 2014 Jan;71(1):1-20. doi: 10.1007/s00018-013-1310-8. Epub 2013 Mar 19. Cell Mol Life Sci. 2014. PMID: 23508806 Free PMC article. Review.
-
Regenerating 1 and 3b gene expression in the pancreas of type 2 diabetic Goto-Kakizaki (GK) rats.PLoS One. 2014 Feb 26;9(2):e90045. doi: 10.1371/journal.pone.0090045. eCollection 2014. PLoS One. 2014. PMID: 24587207 Free PMC article.
References
-
- Watanabe T., Yonekura H., Terazono K., Yamamoto H., Okamoto H. (1990) Complete nucleotide sequence of human reg gene and its expression in normal and tumoral tissues. The reg protein, pancreatic stone protein, and pancreatic thread protein are one and the same product of the gene. J. Biol. Chem. 265, 7432–7439 - PubMed
-
- Laurine E., Manival X., Montgelard C., Bideau C., Bergé-Lefranc J. L., Erard M., Verdier J. M. (2005) PAP IB, a new member of the Reg gene family: cloning, expression, structural properties, and evolution by gene duplication. Biochim. Biophys. Acta 1727, 177–187 - PubMed
-
- De Caro A., Lohse J., Sarles H. (1979) Characterization of a protein isolated from pancreatic calculi of men suffering from chronic calcifying pancreatitis. Biochem. Biophys. Res. Commun. 87, 1176–1182 - PubMed
-
- Bimmler D., Graf R., Scheele G. A., Frick T. W. (1997) Pancreatic stone protein (lithostathine), a physiologically relevant pancreatic calcium carbonate crystal inhibitor? J. Biol. Chem. 272, 3073–3082 - PubMed
-
- Geider S., Baronnet A., Cerini C., Nitsche S., Astier J. P., Michel R., Boistelle R., Berland Y., Dagorn J. C., Verdier J. M. (1996) Pancreatic lithostathine as a calcite habit modifier. J. Biol. Chem. 271, 26302–26306 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

