Sp1 expression regulates lung tumor progression
- PMID: 22158040
- PMCID: PMC3432230
- DOI: 10.1038/onc.2011.568
Sp1 expression regulates lung tumor progression
Abstract
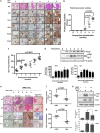
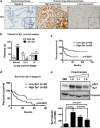
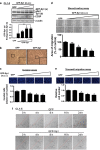
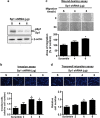
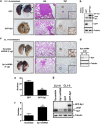
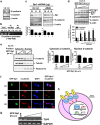
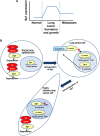
The role of specificity protein 1 (Sp1) in controlling gene expression in lung tumor development and metastasis is not well understood. In this study, we showed that the Sp1 level was highly increased and required for lung tumor growth in transgenic mice bearing Kras-induced lung tumors under the control of doxycycline. Furthermore, the Sp1 level was highly upregulated in lung adenocarcinoma cells with low invasiveness and in patients with stage I lung cancer. We also demonstrated that Sp1 was downregulated in lung adenocarcinoma cells with high invasiveness and in patients with stage IV lung adenocarcinoma. Moreover, Sp1 inversely regulated migration, invasion and metastasis of lung adenocarcinoma cells in vivo. In addition, a decrease in the Sp1 level in highly invasive lung adenocarcinoma cells resulted from instability of the Sp1 protein. Furthermore, overexpression of Sp1 in highly invasive lung adenocarcinoma cells increased expression of E-cadherin, a suppressor of metastasis, and attenuated the translocation of β-catenin into the cellular nucleus that leads to tumor malignancy. Taken together, Sp1 level accumulated strongly in early stage and then declined in late stage, which is important for lung cancer cell proliferation and metastasis during tumorigenesis.
Figures

Similar articles
-
The long non-coding RNA HNF1A-AS1 regulates proliferation and metastasis in lung adenocarcinoma.Oncotarget. 2015 Apr 20;6(11):9160-72. doi: 10.18632/oncotarget.3247. Oncotarget. 2015. PMID: 25863539 Free PMC article.
-
Betulinic acid decreases specificity protein 1 (Sp1) level via increasing the sumoylation of sp1 to inhibit lung cancer growth.Mol Pharmacol. 2012 Dec;82(6):1115-28. doi: 10.1124/mol.112.078485. Epub 2012 Sep 5. Mol Pharmacol. 2012. PMID: 22956772
-
Sp1-mediated microRNA-182 expression regulates lung cancer progression.Oncotarget. 2014 Feb 15;5(3):740-53. doi: 10.18632/oncotarget.1608. Oncotarget. 2014. PMID: 24519909 Free PMC article.
-
[Research progress on relationship between transcription factor Sp1 and tumor].Zhejiang Da Xue Xue Bao Yi Xue Ban. 2010 Mar;39(2):215-20. doi: 10.3785/j.issn.1008-9292.2010.02.018. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2010. PMID: 20387253 Review. Chinese.
-
[The high-grade growth pattern of invasive lung adenocarcinoma: an update].Zhonghua Bing Li Xue Za Zhi. 2024 Nov 8;53(11):1162-1167. doi: 10.3760/cma.j.cn112151-20240222-00112. Zhonghua Bing Li Xue Za Zhi. 2024. PMID: 39482062 Review. Chinese.
Cited by
-
miR-502-5p inhibits the proliferation, migration and invasion of gastric cancer cells by targeting SP1.Oncol Lett. 2020 Sep;20(3):2757-2762. doi: 10.3892/ol.2020.11808. Epub 2020 Jul 3. Oncol Lett. 2020. PMID: 32782592 Free PMC article.
-
Decreased expression level and DNA-binding activity of specificity protein 1 via cyclooxygenase-2 inhibition antagonizes radiation resistance, cell migration and invasion in radiation-resistant lung cancer cells.Oncol Lett. 2018 Sep;16(3):3029-3037. doi: 10.3892/ol.2018.9035. Epub 2018 Jun 28. Oncol Lett. 2018. PMID: 30127893 Free PMC article.
-
MMP7-mediated cleavage of nucleolin at Asp255 induces MMP9 expression to promote tumor malignancy.Oncogene. 2015 Feb 12;34(7):826-37. doi: 10.1038/onc.2014.22. Epub 2014 Mar 17. Oncogene. 2015. PMID: 24632608 Clinical Trial.
-
Specificity protein (Sp) transcription factors and metformin regulate expression of the long non-coding RNA HULC.Oncotarget. 2015 Sep 22;6(28):26359-72. doi: 10.18632/oncotarget.4560. Oncotarget. 2015. PMID: 26317792 Free PMC article.
-
The enhancement of combination of berberine and metformin in inhibition of DNMT1 gene expression through interplay of SP1 and PDPK1.J Cell Mol Med. 2018 Jan;22(1):600-612. doi: 10.1111/jcmm.13347. Epub 2017 Aug 25. J Cell Mol Med. 2018. PMID: 28840963 Free PMC article.
References
-
- Black AR, Black JD, Azizkhan-Clifford J. Sp1 and kruppel-like factor family of transcription factors in cell growth regulation and cancer. J Cell Physiol. 2001;188:143–160. - PubMed
-
- Bremnes RM, Veve R, Hirsch FR, Franklin WA. The E-cadherin cell-cell adhesion complex and lung cancer invasion, metastasis, and prognosis. Lung Cancer. 2002;36:115–124. - PubMed
-
- Chang HC, Liu LT, Hung WC. Involvement of histone deacetylation in ras-induced down-regulation of the metastasis suppressor RECK. Cell Signal. 2004;16:675–679. - PubMed
-
- Chao YC, Pan SH, Yang SC, Yu SL, Che TF, Lin CW, et al. Claudin-1 is a metastasis suppressor and correlates with clinical outcome in lung adenocarcinoma. Am J Respir Crit Care Med. 2009;179:123–133. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

