A nonreplicating subunit vaccine protects mice against lethal Ebola virus challenge
- PMID: 22143779
- PMCID: PMC3251076
- DOI: 10.1073/pnas.1117715108
A nonreplicating subunit vaccine protects mice against lethal Ebola virus challenge
Abstract
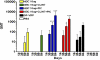
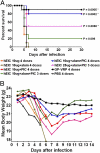
Ebola hemorrhagic fever is an acute and often deadly disease caused by Ebola virus (EBOV). The possible intentional use of this virus against human populations has led to design of vaccines that could be incorporated into a national stockpile for biological threat reduction. We have evaluated the immunogenicity and efficacy of an EBOV vaccine candidate in which the viral surface glycoprotein is biomanufactured as a fusion to a monoclonal antibody that recognizes an epitope in glycoprotein, resulting in the production of Ebola immune complexes (EICs). Although antigen-antibody immune complexes are known to be efficiently processed and presented to immune effector cells, we found that codelivery of the EIC with Toll-like receptor agonists elicited a more robust antibody response in mice than did EIC alone. Among the compounds tested, polyinosinic:polycytidylic acid (PIC, a Toll-like receptor 3 agonist) was highly effective as an adjuvant agent. After vaccinating mice with EIC plus PIC, 80% of the animals were protected against a lethal challenge with live EBOV (30,000 LD(50) of mouse adapted virus). Surviving animals showed a mixed Th1/Th2 response to the antigen, suggesting this may be important for protection. Survival after vaccination with EIC plus PIC was statistically equivalent to that achieved with an alternative viral vector vaccine candidate reported in the literature. Because nonreplicating subunit vaccines offer the possibility of formulation for cost-effective, long-term storage in biothreat reduction repositories, EIC is an attractive option for public health defense measures.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Ebolavirus Glycoprotein Fc Fusion Protein Protects Guinea Pigs against Lethal Challenge.PLoS One. 2016 Sep 13;11(9):e0162446. doi: 10.1371/journal.pone.0162446. eCollection 2016. PLoS One. 2016. PMID: 27622456 Free PMC article.
-
Distinct Immunogenicity and Efficacy of Poxvirus-Based Vaccine Candidates against Ebola Virus Expressing GP and VP40 Proteins.J Virol. 2018 May 14;92(11):e00363-18. doi: 10.1128/JVI.00363-18. Print 2018 Jun 1. J Virol. 2018. PMID: 29514907 Free PMC article.
-
Intradermal immunization by Ebola virus GP subunit vaccines using microneedle patches protects mice against lethal EBOV challenge.Sci Rep. 2018 Jul 25;8(1):11193. doi: 10.1038/s41598-018-29135-w. Sci Rep. 2018. PMID: 30046140 Free PMC article.
-
Ebola virus vaccines: an overview of current approaches.Expert Rev Vaccines. 2014 Apr;13(4):521-31. doi: 10.1586/14760584.2014.885841. Epub 2014 Feb 27. Expert Rev Vaccines. 2014. PMID: 24575870 Free PMC article. Review.
-
Plant-based vaccine research development against viral diseases with emphasis on Ebola virus disease: A review study.Curr Opin Pharmacol. 2021 Oct;60:261-267. doi: 10.1016/j.coph.2021.08.001. Epub 2021 Sep 1. Curr Opin Pharmacol. 2021. PMID: 34481336 Review.
Cited by
-
An adenovirus serotype 2-vectored ebolavirus vaccine generates robust antibody and cell-mediated immune responses in mice and rhesus macaques.Emerg Microbes Infect. 2018 Jun 6;7(1):101. doi: 10.1038/s41426-018-0102-5. Emerg Microbes Infect. 2018. PMID: 29872043 Free PMC article.
-
Use of the Syrian hamster as a new model of ebola virus disease and other viral hemorrhagic fevers.Viruses. 2012 Dec 14;4(12):3754-84. doi: 10.3390/v4123754. Viruses. 2012. PMID: 23242370 Free PMC article. Review.
-
Current ebola vaccines.Expert Opin Biol Ther. 2012 Jul;12(7):859-72. doi: 10.1517/14712598.2012.685152. Epub 2012 May 5. Expert Opin Biol Ther. 2012. PMID: 22559078 Free PMC article. Review.
-
Development of Antibody Therapeutics against Flaviviruses.Int J Mol Sci. 2017 Dec 25;19(1):54. doi: 10.3390/ijms19010054. Int J Mol Sci. 2017. PMID: 29295568 Free PMC article. Review.
-
Generation and analysis of novel plant-derived antibody-based therapeutic molecules against West Nile virus.PLoS One. 2014 Mar 27;9(3):e93541. doi: 10.1371/journal.pone.0093541. eCollection 2014. PLoS One. 2014. PMID: 24675995 Free PMC article.
References
-
- Feldmann H, Klenk HD. Marburg and Ebola viruses. Adv Virus Res. 1996;47:1–52. - PubMed
-
- Jaax N, et al. Transmission of Ebola virus (Zaire strain) to uninfected control monkeys in a biocontainment laboratory. Lancet. 1995;346:1669–1671. - PubMed
-
- Baize S, et al. Defective humoral responses and extensive intravascular apoptosis are associated with fatal outcome in Ebola virus-infected patients. Nat Med. 1999;5:423–426. - PubMed
-
- Warfield KL, et al. Induction of humoral and CD8+ T cell responses are required for protection against lethal Ebola virus infection. J Immunol. 2005;175:1184–1191. - PubMed
-
- Wilson JA, et al. Epitopes involved in antibody-mediated protection from Ebola virus. Science. 2000;287:1664–1666. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

