Sublingual immunization with M2-based vaccine induces broad protective immunity against influenza
- PMID: 22140491
- PMCID: PMC3227615
- DOI: 10.1371/journal.pone.0027953
Sublingual immunization with M2-based vaccine induces broad protective immunity against influenza
Abstract
Background: The ectodomain of matrix protein 2 (M2e) of influenza A virus is a rationale target antigen candidate for the development of a universal vaccine against influenza as M2e undergoes little sequence variation amongst human influenza A strains. Vaccine-induced M2e-specific antibodies (Abs) have been shown to display significant cross-protective activity in animal models. M2e-based vaccine constructs have been shown to be more protective when administered by the intranasal (i.n.) route than after parenteral injection. However, i.n. administration of vaccines poses rare but serious safety issues associated with retrograde passage of inhaled antigens and adjuvants through the olfactory epithelium. In this study, we examined whether the sublingual (s.l.) route could serve as a safe and effective alternative mucosal delivery route for administering a prototype M2e-based vaccine. The mechanism whereby s.l. immunization with M2e vaccine candidate induces broad protection against infection with different influenza virus subtypes was explored.
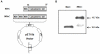
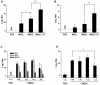
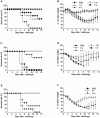
Methods and results: A recombinant M2 protein with three tandem copies of the M2e (3M2eC) was expressed in Escherichia coli. Parenteral immunizations of mice with 3M2eC induced high levels of M2e-specific serum Abs but failed to provide complete protection against lethal challenge with influenza virus. In contrast, s.l. immunization with 3M2eC was superior for inducing protection in mice. In the latter animals, protection was associated with specific Ab responses in the lungs.
Conclusions: The results demonstrate that s.l. immunization with 3M2eC vaccine induced airway mucosal immune responses along with broad cross-protective immunity to influenza. These findings may contribute to the understanding of the M2-based vaccine approach to control epidemic and pandemic influenza infections.
Conflict of interest statement
Figures
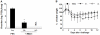
Similar articles
-
Roles of adjuvant and route of vaccination in antibody response and protection engendered by a synthetic matrix protein 2-based influenza A virus vaccine in the mouse.Virol J. 2007 Oct 31;4:118. doi: 10.1186/1743-422X-4-118. Virol J. 2007. PMID: 17974006 Free PMC article.
-
Intranasal adenovirus-vectored vaccine for induction of long-lasting humoral immunity-mediated broad protection against influenza in mice.J Virol. 2014 Sep 1;88(17):9693-703. doi: 10.1128/JVI.00823-14. Epub 2014 Jun 11. J Virol. 2014. PMID: 24920793 Free PMC article.
-
Protection against homo and hetero-subtypic influenza A virus by optimized M2e DNA vaccine.Emerg Microbes Infect. 2019;8(1):45-54. doi: 10.1080/22221751.2018.1558962. Emerg Microbes Infect. 2019. PMID: 30866759 Free PMC article.
-
Influenza A viruses: why focusing on M2e-based universal vaccines.Virus Genes. 2011 Feb;42(1):1-8. doi: 10.1007/s11262-010-0547-7. Epub 2010 Nov 17. Virus Genes. 2011. PMID: 21082230 Review.
-
Development of universal influenza vaccines based on influenza virus M and NP genes.Infection. 2014 Apr;42(2):251-62. doi: 10.1007/s15010-013-0546-4. Epub 2013 Nov 1. Infection. 2014. PMID: 24178189 Review.
Cited by
-
Oral mucosa immunity: ultimate strategy to stop spreading of pandemic viruses.Front Immunol. 2023 Oct 19;14:1220610. doi: 10.3389/fimmu.2023.1220610. eCollection 2023. Front Immunol. 2023. PMID: 37928529 Free PMC article. Review.
-
The novel immunobiotic Clostridium butyricum S-45-5 displays broad-spectrum antiviral activity in vitro and in vivo by inducing immune modulation.Front Immunol. 2023 Oct 10;14:1242183. doi: 10.3389/fimmu.2023.1242183. eCollection 2023. Front Immunol. 2023. PMID: 37881429 Free PMC article.
-
M2e-Based Influenza Vaccines with Nucleoprotein: A Review.Vaccines (Basel). 2021 Jul 4;9(7):739. doi: 10.3390/vaccines9070739. Vaccines (Basel). 2021. PMID: 34358155 Free PMC article. Review.
-
Intranasal Vaccine Delivery Technology for Respiratory Tract Disease Application with a Special Emphasis on Pneumococcal Disease.Vaccines (Basel). 2021 Jun 2;9(6):589. doi: 10.3390/vaccines9060589. Vaccines (Basel). 2021. PMID: 34199398 Free PMC article. Review.
-
Influenza Chimeric Protein (3M2e-3HA2-NP) Adjuvanted with PGA/Alum Confers Cross-Protection against Heterologous Influenza A Viruses.J Microbiol Biotechnol. 2021 Feb 28;31(2):304-316. doi: 10.4014/jmb.2011.11029. J Microbiol Biotechnol. 2021. PMID: 33263336 Free PMC article.
References
-
- Steinhauer DA, Skehel JJ. Genetics of influenza viruses. Annu Rev Genet. 2002;36:305–332. - PubMed
-
- Ada G, Jones P. The immune response to influenza infection. Curr Top Microbiol Immunol. 1986;128:1–54. - PubMed
-
- Holsinger L, Lamb R. Influenza virus M2 integral membrane protein is a homotetramer stabilized by formation of disulfide bonds. Virology. 1991;183:32–43. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

