Skin mast cells protect mice against vaccinia virus by triggering mast cell receptor S1PR2 and releasing antimicrobial peptides
- PMID: 22140255
- PMCID: PMC3244574
- DOI: 10.4049/jimmunol.1101703
Skin mast cells protect mice against vaccinia virus by triggering mast cell receptor S1PR2 and releasing antimicrobial peptides
Abstract
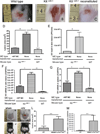
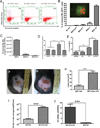
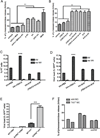
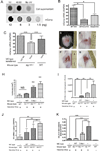
Mast cells (MCs) are well-known effectors of allergic reactions and are considered sentinels in the skin and mucosa. In addition, through their production of cathelicidin, MCs have the capacity to oppose invading pathogens. We therefore hypothesized that MCs could act as sentinels in the skin against viral infections using antimicrobial peptides. In this study, we demonstrate that MCs react to vaccinia virus (VV) and degranulate using a membrane-activated pathway that leads to antimicrobial peptide discharge and virus inactivation. This finding was supported using a mouse model of viral infection. MC-deficient (Kit(wsh-/-)) mice were more susceptible to skin VV infection than the wild type animals, whereas Kit(wsh-/-) mice reconstituted with MCs in the skin showed a normal response to VV. Using MCs derived from mice deficient in cathelicidin antimicrobial peptide, we showed that antimicrobial peptides are one important antiviral granule component in in vivo skin infections. In conclusion, we demonstrate that MC presence protects mice from VV skin infection, MC degranulation is required for protecting mice from VV, neutralizing Ab to the L1 fusion entry protein of VV inhibits degranulation apparently by preventing S1PR2 activation by viral membrane lipids, and antimicrobial peptide release from MC granules is necessary to inactivate VV infectivity.
Figures
Similar articles
-
Commensal bacteria lipoteichoic acid increases skin mast cell antimicrobial activity against vaccinia viruses.J Immunol. 2012 Aug 15;189(4):1551-8. doi: 10.4049/jimmunol.1200471. Epub 2012 Jul 6. J Immunol. 2012. PMID: 22772452 Free PMC article.
-
Mast cell cathelicidin antimicrobial peptide prevents invasive group A Streptococcus infection of the skin.J Immunol. 2008 Jun 1;180(11):7565-73. doi: 10.4049/jimmunol.180.11.7565. J Immunol. 2008. PMID: 18490758 Free PMC article.
-
Cytokine milieu of atopic dermatitis skin subverts the innate immune response to vaccinia virus.Immunity. 2006 Mar;24(3):341-8. doi: 10.1016/j.immuni.2006.02.006. Immunity. 2006. PMID: 16546102
-
Emerging Roles for MAS-Related G Protein-Coupled Receptor-X2 in Host Defense Peptide, Opioid, and Neuropeptide-Mediated Inflammatory Reactions.Adv Immunol. 2017;136:123-162. doi: 10.1016/bs.ai.2017.06.002. Epub 2017 Jul 24. Adv Immunol. 2017. PMID: 28950944 Review.
-
Antimicrobial peptides: effectors of innate immunity in the skin.Adv Dermatol. 2005;21:357-74. doi: 10.1016/j.yadr.2005.07.001. Adv Dermatol. 2005. PMID: 16350450 Review.
Cited by
-
Eosinophils: The unsung heroes in cancer?Oncoimmunology. 2017 Nov 13;7(2):e1393134. doi: 10.1080/2162402X.2017.1393134. eCollection 2018. Oncoimmunology. 2017. PMID: 29308325 Free PMC article. Review.
-
Innate immunity and the role of the antimicrobial peptide cathelicidin in inflammatory skin disease.Drug Discov Today Dis Mech. 2013 Dec 1;10(3-4):e79-e82. doi: 10.1016/j.ddmec.2013.01.001. Drug Discov Today Dis Mech. 2013. PMID: 24489580 Free PMC article.
-
Interaction of primary mast cells with Borrelia burgdorferi (sensu stricto): role in transmission and dissemination in C57BL/6 mice.Parasit Vectors. 2017 Jun 27;10(1):313. doi: 10.1186/s13071-017-2243-0. Parasit Vectors. 2017. PMID: 28655322 Free PMC article.
-
Mast cells and influenza a virus: association with allergic responses and beyond.Front Immunol. 2015 May 18;6:238. doi: 10.3389/fimmu.2015.00238. eCollection 2015. Front Immunol. 2015. PMID: 26042121 Free PMC article. Review.
-
Mast Cell Responses to Viruses and Pathogen Products.Int J Mol Sci. 2019 Aug 30;20(17):4241. doi: 10.3390/ijms20174241. Int J Mol Sci. 2019. PMID: 31480219 Free PMC article. Review.
References
-
- Galli SJ, Nakae S, Tsai M. Mast cells in the development of adaptive immune responses. Nat Immunol. 2005;6:135–142. - PubMed
-
- Matsushima H, Yamada N, Matsue H, Shimada S. TLR3-, TLR7-, and TLR9-mediated production of proinflammatory cytokines and chemokines from murine connective tissue type skin-derived mast cells but not from bone marrow-derived mast cells. J Immunol. 2004;173:531–541. - PubMed
-
- Fehrenbach K, Port F, Grochowy G, Kalis C, Bessler W, Galanos C, Krystal G, Freudenberg M, Huber M. Stimulation of mast cells via FcvarepsilonR1 and TLR2: the type of ligand determines the outcome. Mol Immunol. 2007;44:2087–2094. - PubMed
-
- Krishnaswamy G, Kelley J, Johnson D, Youngberg G, Stone W, Huang SK, Bieber J, Chi DS. The human mast cell: functions in physiology and disease. Front Biosci. 2001;6:D1109–D1127. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

