Down-regulation of DcR2 sensitizes androgen-dependent prostate cancer LNCaP cells to TRAIL-induced apoptosis
- PMID: 22136382
- PMCID: PMC3286382
- DOI: 10.1186/1475-2867-11-42
Down-regulation of DcR2 sensitizes androgen-dependent prostate cancer LNCaP cells to TRAIL-induced apoptosis
Abstract
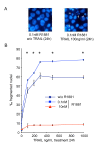
Background: Dysregulation of many apoptotic related genes and androgens are critical in the development, progression, and treatment of prostate cancer. The differential sensitivity of tumour cells to TRAIL-induced apoptosis can be mediated by the modulation of surface TRAIL receptor expression related to androgen concentration. Our previous results led to the hypothesis that downregulation of TRAIL-decoy receptor DcR2 expression following androgen deprivation would leave hormone sensitive normal prostate cells vulnerable to the cell death signal generated by TRAIL via its pro-apoptotic receptors. We tested this hypothesis under pathological conditions by exploring the regulation of TRAIL-induced apoptosis related to their death and decoy receptor expression, as also to hormonal concentrations in androgen-sensitive human prostate cancer, LNCaP, cells.
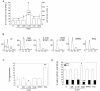
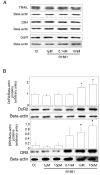
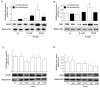
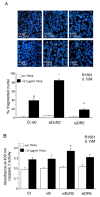
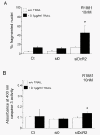
Results: In contrast to androgen-insensitive PC3 cells, decoy (DcR2) and death (DR5) receptor protein expression was correlated with hormone concentrations and TRAIL-induced apoptosis in LNCaP cells. Silencing of androgen-sensitive DcR2 protein expression by siRNA led to a significant increase in TRAIL-mediated apoptosis related to androgen concentration in LNCaP cells.
Conclusions: The data support the hypothesis that hormone modulation of DcR2 expression regulates TRAIL-induced apoptosis in LNCaP cells, giving insight into cell death induction in apoptosis-resistant hormone-sensitive tumour cells from prostate cancer. TRAIL action and DcR2 expression modulation are potentially of clinical value in advanced tumour treatment.
Figures

Similar articles
-
TNF-alpha-related apoptosis-inducing ligand decoy receptor DcR2 is targeted by androgen action in the rat ventral prostate.J Cell Physiol. 2006 Mar;206(3):709-17. doi: 10.1002/jcp.20520. J Cell Physiol. 2006. PMID: 16245307
-
DcR2 (TRAIL-R4) siRNA and adenovirus delivery of TRAIL (Ad5hTRAIL) break down in vitro tumorigenic potential of prostate carcinoma cells.Cancer Gene Ther. 2007 Dec;14(12):976-84. doi: 10.1038/sj.cgt.7701087. Epub 2007 Sep 14. Cancer Gene Ther. 2007. PMID: 17853923
-
Sensitivity of prostate cells to TRAIL-induced apoptosis increases with tumor progression: DR5 and caspase 8 are key players.Prostate. 2006 Jun 15;66(9):987-95. doi: 10.1002/pros.20421. Prostate. 2006. PMID: 16541419
-
E3 ubiquitin ligases and deubiquitinases as modulators of TRAIL-mediated extrinsic apoptotic signaling pathway.BMB Rep. 2019 Feb;52(2):119-126. doi: 10.5483/BMBRep.2019.52.2.011. BMB Rep. 2019. PMID: 30638181 Free PMC article. Review.
-
Down-regulation of intracellular anti-apoptotic proteins, particularly c-FLIP by therapeutic agents; the novel view to overcome resistance to TRAIL.J Cell Physiol. 2018 Oct;233(10):6470-6485. doi: 10.1002/jcp.26585. Epub 2018 May 9. J Cell Physiol. 2018. PMID: 29741767 Review.
Cited by
-
Poly(ADP-ribose) polymerase-13 and RNA regulation in immunity and cancer.Trends Mol Med. 2015 Jun;21(6):373-84. doi: 10.1016/j.molmed.2015.03.002. Epub 2015 Apr 4. Trends Mol Med. 2015. PMID: 25851173 Free PMC article. Review.
-
The role of high cell density in the promotion of neuroendocrine transdifferentiation of prostate cancer cells.Mol Cancer. 2014 May 20;13:113. doi: 10.1186/1476-4598-13-113. Mol Cancer. 2014. PMID: 24884804 Free PMC article.
-
Should We Keep Walking along the Trail for Pancreatic Cancer Treatment? Revisiting TNF-Related Apoptosis-Inducing Ligand for Anticancer Therapy.Cancers (Basel). 2018 Mar 18;10(3):77. doi: 10.3390/cancers10030077. Cancers (Basel). 2018. PMID: 29562636 Free PMC article. Review.
-
Senescent cells: an emerging target for diseases of ageing.Nat Rev Drug Discov. 2017 Oct;16(10):718-735. doi: 10.1038/nrd.2017.116. Epub 2017 Jul 21. Nat Rev Drug Discov. 2017. PMID: 28729727 Free PMC article. Review.
-
Synergistic Effect of Subtoxic-dose Cisplatin and TRAIL to Mediate Apoptosis by Down-regulating Decoy Receptor 2 and Up-regulating Caspase-8, Caspase-9 and Bax Expression on NCI-H460 and A549 Cells.Iran J Basic Med Sci. 2013 May;16(5):710-8. Iran J Basic Med Sci. 2013. PMID: 23826494 Free PMC article.
References
-
- Zornig M, Hueber AO, Baum W, Evan G. Apoptosis regulators and their role in tumorigenesis. Biochem Biophys Acta. 2001;1551:F1–F37. - PubMed
LinkOut - more resources
Full Text Sources

